- Home
- Equipment
- north america
- sars cov 2
Refine by
Sars Cov 2 Equipment Supplied In North America
218 equipment items found
Distributed by:BioFlyte, Inc. based inAlbuquerque, NEW MEXICO (USA)
Indoor air sampling for on-site and near-real-time detection of airborne coronavirus. A fast and cost-effective COVID-19 workplace safety solution for: Schools, Factories and food processing plants, Restaurants and entertainment venues, Prisons and detention centers, Retail ...
Manufactured by:Immudex ApS based inVirum, DENMARK
Reliable Identification of SARS-CoV-2-Specific T-Cell Responses. T Cells Take the Lead in COVID-19. A deeper understanding of viral-specific T-cell responses to COVID-19 is needed to explore vaccine responses, long-term immunity, predict patients at risk, and even prepare for the next pandemic. T-cell responses are believed to be important for ...
Manufactured by:AIVITA Biomedical, Inc. based in, CALIFORNIA (USA)
AIVITA Biomedical is using its patient-specific vaccine platform to develop a rapidly scalable vaccine candidate, AV-COVID-19, for the novel coronavirus (SARS-CoV-2). AIVITA’s personalized vaccine begins with a basic blood draw, from which the recipient’s own immune cells are extracted and loaded with ...
Manufactured by:Genscript based inPiscataway, NEW JERSEY (USA)
SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) also known as 2019-nCoV (2019 Novel Coronavirus) is a virus that causes illnesses ranging from the common cold to severe diseases. The spike protein mutation N501Y is one of six key contact residues within the receptor-binding domain (RBD) and has been identified as ...
Manufactured by:TBG Diagnostics Limited (TDL) based inMelbourne, AUSTRALIA
ExProbe SARS-CoV-2 Testing Kit is used for qualitative detection of the RdRP, N and E genes of SARS-CoV-2 in pharyngeal swabs and sputum samples. The test results of this kit can qualitatively determine whether the suspected patient is infected with 2019 novel coronavirus emerging pneumonia ...
Manufactured by:Gold Standard Diagnostics based inDavis, CALIFORNIA (USA)
CE marked, rapid and reliable RT-PCR assay for the differentiation of Omicron from other VOCs (Beta, Gamma, and Delta). GSD NovaType IV SARS-CoV-2 specifically identifies S gene mutations K417N, E484K, and L452R. Screening for S gene mutations K417N and L452R allows for active surveillance of variants Omicron and Delta, ...
Manufactured by:Thermo Fisher Scientific based inWaltham, MASSACHUSETTS (USA)
Choose from a menu of verified real-time PCR assays to build your own custom Applied Biosystems TaqMan SARS-CoV-2 Mutation Panel, and get the results you need today, while preparing you for what’s to come. This scalable solution lets you run a few or hundreds of samples to identify one or many mutations—all on your current real-time ...
by:Diasorin based inSALUGGIA, ITALY
DiaSorin strives to improve health-care around the globe through the development of innovative diagnostic solutions that can lead to better patient outcomes and provide economic benefits to the healthcare system. Thanks to its team of dedicated scientists DiaSorin has been able to promptly deliver diagnostic solutions to manage the COVID-19 pandemic ...
Manufactured by:Advansta Inc. based inSan Jose, CALIFORNIA (USA)
In December of 2019, the SARS-CoV-2 virus, which causes the disease COVID-19, was first detected in Wuhan, China. The SARS-CoV-2 virus is a single-stranded RNA coronavirus that causes respiratory infections. The rapid spread of COVID-19 has resulted in a global pandemic which has caused an ...
Manufactured by:Hologic, Inc. based inMarlborough, MASSACHUSETTS (USA)
The emergence of SARS-CoV-2 was unforeseen, and the worldwide outbreak of COVID-19 has already impacted millions. In response to the desperate public need for rapid high-volume testing we developed SARS-CoV-2 assays on both our Panther® and Panther Fusion® systems.* Both assays enable ...
Manufactured by:Integrated DNA Technologies, Inc. based inCoralville, IOWA (USA)
The US Centers for Disease Control and Prevention (CDC) has designed RT-PCR assays and published a protocol for the detection of the 2019-nCoV. IDT provides primers and probes for these CDC assays for the identification of virus. The primers and probes are manufactured at IDT under ISO 13485:2016 conditions and are available for research use only (RUO). The kits include primers and 5’ FAM / ...
Manufactured by:Adaptive Biotechnologies based inSeattle, WASHINGTON (USA)
These antibodies may be used therapeutically to treat someone currently fighting the disease and provide passive immunity to people who have recently been exposed to ...
Manufactured by:AstraZeneca based inCambridge, UNITED KINGDOM
IVX-411 targets SARS-CoV-2, the coronavirus that causes COVID-19. Developed using cutting-edge structure-based design techniques at the Institute for Protein Design at the UW School of Medicine, IVX-411, our lead vaccine candidate for COVID-19, is a self-assembling protein nanoparticle that displays 60 copies of the ...
by:Thermo Fisher Scientific, LIMS & Laboratory Software based inPhiladelphia, PENNSYLVANIA (USA)
For universities, businesses, and communities eager to reconnect and resume everyday activities, surveillance testing of the SARS-CoV-2 virus is essential to helping monitor population level occurrence. Our PCR testing research solution enables comprehensive, high-frequency surveillance testing at a scale to support your entire ...
Manufactured by:Anchor Molecular based inPleasanton, CALIFORNIA (USA)
The Anchor SARS-COV-2 Quality Controls contain a non-pathogenic recombinant RNA virus. This allows for the monitoring of the entire testing process, from the extraction of viral RNA to the end result of the nucleic acid amplification. The virus carries known target sequences (Wuhan-Hu-1, NC_045512.2) used in more than 160 molecular tests ...
Manufactured by:BioGX, Inc. based inBirmingham, ALABAMA (USA)
BioGX SARS-CoV-2 Reagents for BD MAX™ is a real time reverse transcriptase, polymerase chain reaction (PCR) assay for use on the BD MAX™ platform for the qualitative detection of the presence of RNA from nucleocapsid phosphoprotein gene (N gene) of the SARS-CoV-2 coronavirus and the ...
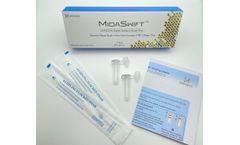
Manufactured by:Nirmidas Biotech, Inc. based inPalo Alto, CALIFORNIA (USA)
Nirmidas Biotech Inc. has developed an antigen test aiming for FDA EUA, MidaSwift™ SARS-CoV-2 Antigen Detection Kit, for the qualitative detection of the Nucleocapsid antigen from SARS-CoV-2. The test is curently for Research Use Only (RUO), for use with anterior nares (lower nostril) swab ...
by:T2 Biosystems, Inc. based inLexington, MASSACHUSETTS (USA)
The T2SARS-CoV-2 Panel, run on the T2Dx® Instrument, is a qualitative reverse transcriptase-polymerase chain reaction (RT-PCR) and T2 Magnetic Resonance (T2MR®) test. This test is designed for the direct detection of nucleic acid from SARS-CoV-2 in upper respiratory specimens in transport media from ...
Manufactured by:Lumos Diagnostics based inSarasota, FLORIDA (USA)
CoviDx™ SARS-CoV-2 Rapid Antigen Test is a lateral flow assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasal swabs and nasopharyngeal swabs from patients suspected of a Coronavirus-19 (COVID-19) infection by their healthcare provider ...
Manufactured by:Gold Standard Diagnostics based inDavis, CALIFORNIA (USA)
The GSD NovaPrime® TSP SARS-CoV-2 (COVID-19) is intended for the qualitative determination of SARS-CoV-2 genomic RNA in several respiratory sample types to aid in the diagnosis of COVID-19. This product represents an improvement in comparison to its former version GSD NovaPrime® ...