- Home
- Equipment
- north america
- skin preparation
Refine by
Skin Preparation Equipment Supplied In North America
18 equipment items found
Manufactured by:Adhezion Biomedical, LLC based inWyomissing, PENNSYLVANIA (USA)
FloraSeal® Microbial Sealant is a sterile, liquid microbial sealant intended for use after topical operative skin preparations with standard surgical draping and prior to a surgical incision. The product is used to reduce the risk of skin flora contamination throughout a surgical ...
Manufactured by:S&W Healthcare Corporation based inBrooksville, FLORIDA (USA)
Easiprep Strips are a revolutionary ECG Electrode skin preparation product! Simply apply one self adhesive backed strip to your finger tip and lightly abrade your patient’s skin surface before applying your ECG Electrodes. Quick, Easy, Fast preparation for clearer, quicker tracings! ...
Manufactured by:Zoetis Services LLC based inParsippany, NEW JERSEY (USA)
Iovone Solution is a povidone iodine solution for surgical skin preparation and wound ...
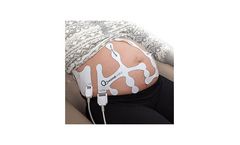
Manufactured by:Mindchild Medical, Inc. based inNorth Andover, MASSACHUSETTS (USA)
The MERIDIAN M110 Disposable Electrode Patch eliminates the need for skin preparation before use while providing outstanding performance and reliability across the full range of patient BMI anticipated during labor and delivery. The MERIDIAN M110 Disposable Electrode Patch replaces the fetal scalp electrode, intrauterine pressure catheter, Dopper, and TOCO ...
Manufactured by:Sol-Millennium Medical Group based inSuwanee, GEORGIA (US) (USA)
Sterile non-woven pad, impregnated with 70% Isopropyl Alcohol formulation. Intended for skin disinfection and to be used for preparation of the skin prior an injection. Individually foil wrapped for convenience. The four-layer wrapper provides air-tight seal which prevents leakage and drying out. Sterile and non sterile versions available. ...
Manufactured by:Medsource Labs based inChanhassen, MINNESOTA (USA)
Alcohol Prep Pads contain 70% Isopropyl alcohol for optimum anti-bacterial action. PVP Prep Pads contain 10% povidone Iodine for optimum anti-bacterial ...
Manufactured by:NxStage Medical, Inc. based inLawrence, MASSACHUSETTS (USA)
ButtonHole AV Fistula Needles with SteriPick are used for Buttonhole or constant-site cannulation. ButtonHole cannulation is the practice of cannulating an AV fistula by going into the exact same spot repeatedly with a dull needle. This technique has been used world wide for 35 years.1 ...
Manufactured by:AVITA Medical based inValencia, CALIFORNIA (USA)
AVITA Medical’s first product, the RECELL® System, is a device that enables healthcare professionals to produce a suspension of Spray-On Skin™ Cells using a small sample of the patient’s own skin for the treatment of acute thermal burns. This suspension contains the cells necessary to regenerate the outer layer of natural, healthy ...
Manufactured by:Trio Ostomy Care, Trademark of Trio Healthcare Ltd based inPrinceton, NEW JERSEY (USA)
The Trio Elite Sting Free Adhesive Remover is formulated to offer an advanced solution for individuals requiring stoma care. It utilizes a patented 100% silicone formulation, ensuring a hypoallergenic and sting-free experience. This remover is specifically designed for ease and effectiveness in removing adhesives commonly used in stoma care, such as base plates, wafers, pastes, and hydrocolloid ...
Manufactured by:Tennant Products based inColleyville, TEXAS (USA)
Pneuma is now N1O1 Nitric Oxide Activating Serum with antioxidants and now offers 30% more applications in each ...
Manufactured by:NovaFlux based inPrinceton, NEW JERSEY (USA)
NanoClean is a liquid solution infused with microfibrils and formulated to fully eradicate contaminants when it is perfused across a surface. This even includes contaminants such as build-up biofilm, which is typically unperturbed by standard cleaning products and processes. NanoClean is currently developed for use in medical equipment, and different formulas can be applied for narrower or wider ...
Manufactured by:Gebauer Company based inCleveland, OHIO (USA)
Gebauer's Pain Ease is a topical anesthetic skin refrigerant (vapocoolant) that begins working in just 4-10 seconds. Pain Ease is FDA-cleared to temporarily control the pain associated with needle procedures and minor surgical ...
Manufactured by:Zoetis Services LLC based inParsippany, NEW JERSEY (USA)
Antiseptic/disinfectant. Wide spectrum of antimicrobial activity and sustained residual effect due to binding with skin proteins. Chlorhex-C is not affected by the presence of blood and organic ...
Manufactured by:Lohmann & Rauscher GmbH & Co. KG (L&R) based inRengsdorf, GERMANY
The Suprasorb C Collagen Wound Dressing has a porous structure with highly pronounced capillary activity, allowing absorption of fluid. This physical property allows continuous absorption of exudate, debris (such as necrotic tissue, fibrin slough) and inflammatory proteases and cytokines. This promotes and accelerates formation of granulation tissue. Migration of fibroblasts is induced and ...
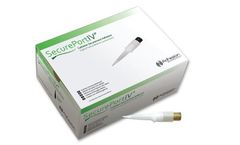
Manufactured by:Adhezion Biomedical, LLC based inWyomissing, PENNSYLVANIA (USA)
The most versatile care and maintenance device in vascular access. Standard of care in vascular access typically involves the use of multiple products for securement and insertion site protection. See how SecurePortIV® Catheter Securement Adhesive is combining multiple patient and caregiver benefits into one ...
Manufactured by:Mindchild Medical, Inc. based inNorth Andover, MASSACHUSETTS (USA)
MindChild Medical has developed the MERIDIAN M110, a highly sensitive and reliable, non-invasive fetal and maternal monitoring technology that displays fetal and maternal heart rate as well as uterine contractions during the course of labor and delivery without signal loss and the need for repositioning due to mother and fetal ...
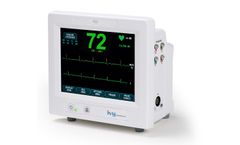
Manufactured by:Ivy Biomedical Systems, Inc based inBranford, CONNECTICUT (USA)
The Model 7800 is Ivy Biomedical System’s 5th generation cardiac trigger monitor used in applications requiring precision ECG R-wave synchronization for gating host systems on and off. The Model 7800 incorporates an intuitive, menu-driven, touch-screen user interface, simplifying operation for clinicians and ...
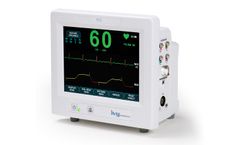
Manufactured by:Ivy Biomedical Systems, Inc based inBranford, CONNECTICUT (USA)
The Model 7810 is Ivy Biomedical System’s newest product introduction providing dual (cardiac and respiratory) gating functionality used in applications requiring precision ECG R-wave and respiratory cycle synchronization for gating host systems on and off. The Model 7810 incorporates an intuitive, menu-driven, touch-screen user interface, simplifying operation for clinicians and ...