- Home
- Equipment
- north america
- test covid 19 kit
Show results for
Refine by
Test Covid 19 Kit Equipment Supplied In North America
26 equipment items found
Manufactured by:DxTerity Diagnostics Inc. based inRancho Dominguez, CALIFORNIA (USA)
COVID-19 is caused by the SARS-CoV-2 virus which is a new virus in humans causing a contagious respiratory illness. COVID-19 can present with a mild to severe illness, although some people infected with COVID-19 may have no symptoms at all. Older adults and people of any age who have underlying medical conditions have a higher risk of severe illness from COVID-19. Serious outcomes of COVID-19 ...
Manufactured by:TBG Diagnostics Limited (TDL) based inMelbourne, AUSTRALIA
The SARS-CoV-2 IgG/IgM Rapid Test Kit is used to qualitatively detect IgG and IgM antibodies of SARS-CoV-2 in human serum, plasma or whole blood. This device is intended to be used by professionals as a screening test and for SARS-CoV-2 research. Any reactive specimen with the SARS-CoV-2 IgG/IgM rapid test kit should be confirmed with alternative testing method(s). ...
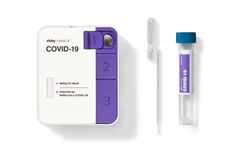
Manufactured by:Visby Medical, Inc. based inSan Jose, CALIFORNIA (USA)
Fast, accurate, and actionable PCR results in under 30 minutes. Instrument-free PCR: Empower on-demand testing, when and where you need ...
Manufactured by:BIOVECTRA based inCharlottetown, PRINCE EDWARD ISLAND (CANADA)
Reagent used for maintaining thiol groups in a reduced state during protein processing, isolation, and denaturing. Incorporated into some leading COVID-19 testing ...
Distributed by:Alban Scientific, Inc. based inSt. Louis, MISSOURI (USA)
QuickVue At-Home OTC COVID-19 Test lets you get rapid results, in the privacy of your own home. Available over-the-counter, everything you need is in the package and taking the test is simple. The test is authorized for home use with self-collected anterior nasal (nares) swab samples in individuals aged 2 and older. This test is also authorized for home use for individuals aged 2 through 14 ...
Manufactured by:Spark Mobile Diagnostics based inJamnagar, INDIA
CoV-Check DIRECT Covid-19 Antigen Test is a rapid immunochromatographic assay that utilizes specific monoclonal antibodies to detect Nucleocapsid protein of SARS-CoV-2 virus in nasopharyngeal swab specimens. This test provides a quick and easy to use test to help in the diagnosis of SARS-CoV-2 infection to humans. ...
by:BioLab Sciences Inc. based inScottsdale, ARIZONA (USA)
BioLab Sciences is distributing Assure COVID-19 test kits. For more information, please click on the buttons below to open up the ...
Manufactured by:TBG Diagnostics Limited (TDL) based inMelbourne, AUSTRALIA
ExProbe SARS-CoV-2 Testing Kit is used for qualitative detection of the RdRP, N and E genes of SARS-CoV-2 in pharyngeal swabs and sputum samples. The test results of this kit can qualitatively determine whether the suspected patient is infected with 2019 novel coronavirus emerging pneumonia infection of unknown causes. ...
Manufactured by:DxTerity Diagnostics Inc. based inRancho Dominguez, CALIFORNIA (USA)
Our SARS-CoV-2 RT-PCR saliva-based test is FDA EUA authorized for at home unsupervised sample collection, making easy for you to take your test in the comfort of your home. Collect your sample at home and then ship it back to our lab. We use state-of-the-art technology to get your accurate results. Results are reported to you through our secure portal. ...
Manufactured by:Anavasi Diagnostics based inSeattle, WASHINGTON (USA)
The AscencioDx COVID-19 Molecular Detector is a rapid, highly accurate, easy-to-use and affordable clinical diagnostic platform used for performing molecular analysis of samples collected from patients in point-of-care (POC) settings utilizing the proprietary AscencioDx COVID-19 Test ...
Manufactured by:BioGX, Inc. based inBirmingham, ALABAMA (USA)
The Xfree™ COVID-19 Direct RT-PCR test is offered lyophilized in a single vial, in the trusted BioGX Sample-Ready™ “Just Add Water” format where no other reagent is required. The test is available for the most ubiquitous real-time PCR platforms already available in thousands of laboratories. The fast and simple workflow is designed to help laboratories to increase their ...
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
Self Test for SARS-CoV-2 Antigen Detection. The CareStart™ COVID-19 Antigen Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigens from SARS-CoV-2. Fast and easy to self-test at anywhere. Easy to interpret the results using mobile application. Qualitatively detect the SARS-CoV-2 nucleocapsid protein. Use for nasal swab specimen. Fast ...
by:GENETWORx, LLC. based inGlen Allen, VIRGINIA (USA)
GENETWORx is your one stop solution for COVID-19 testing. We now offer COVID-19 nasal swab self-collection test kits, in addition to on-site testing and COVID-19 management software. The GENETWORx ...
Distributed by:Labex Canada Inc. based in, ONTARIO (CANADA)
The COVID-19 Vaccine IgG Rapid Test is a lateral flow immunoassay intended for the qualitative detection of antibodies binding to the Receptor Binding Domain (RBD) of the spike protein of the SARS-CoV-2 virus or COVID-19 vaccine in human serum, plasma or whole blood. The kit is intended for ...
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
An immunochromatographic rapid diagnostic test (RDT) intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nasopharyngeal (NP) or anteriornasal (AN) swab specimens. Authorized for use under an Emergency Use Authorization (EUA) and for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate ...
Manufactured by:NOWDiagnostics Inc. based inSpringdale, ARKANSAS (USA)
The ADEXUSD COVID-19 Test is an in vitro lateral-flow immunoassay intended for qualitative detection of total antibodies to SARS-CoV-2 in human venous whole blood (dipotassium EDTA) and plasma (dipotassium EDTA), serum, and fingerstick whole ...
Manufactured by:Lucira Health - Pfizer based inEmeryville, CALIFORNIA (USA)
The Lucira Check It is a molecular or nucleic acid amplification test (NAAT) that provides accurate, at-home results for the detection of the COVID-19 virus. Our tests have an analytical sensitivity (ability to detect the SARS-CoV-2 virus) that is comparable to some of the best performing PCR tests in ...
Distributed by:Labex Canada Inc. based in, ONTARIO (CANADA)
Coronaviruses belong to a large family of potentially harmful RNA viruses. In humans, they are known to be involved mainly in respiratory infections ranging from the common colds to more severe diseases such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The recently discovered coronavirus, SARS-CoV-2, causes coronavirus disease COVID-19, a significant ...
Manufactured by:DxTerity Diagnostics Inc. based inRancho Dominguez, CALIFORNIA (USA)
The DxTerity SARS-CoV-2 (COVID-19) RT-PCR CE saliva-based test provides the convenience of at-home sample collection with results available within 24-72 hours. Our highly-sensitive, gold-standard PCR test has been verified to work with all COVID-19 ...
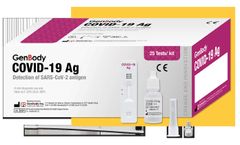
Distributed by:Labex Canada Inc. based in, ONTARIO (CANADA)
Coronaviruses belong to a large family of potentially harmful RNA viruses. In humans, they are known to be involved mainly in respiratory infections ranging from the common colds to more severe diseases such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The recently discovered coronavirus, SARS-CoV-2, causes coronavirus disease COVID-19, a significant ...