- Home
- Equipment
- north america
- vascular abi system
Show results for
Refine by
Vascular Abi System Equipment Supplied In North America
48 equipment items found
Manufactured by:Newman Medical based inArvada, COLORADO (USA)
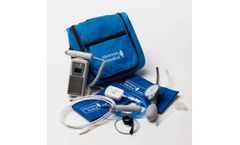
A manual vascular system. The ABI-300 performs lower extremity physiologic arterial exams including: (i) baseline ABI exams & (ii) ABI with Toe (TBI) exams. The ABI-300 comes with simpleABI Software, Doppler with D8 Doppler probe, DPPG probe, PVR, 2x10cm cuffs, 2x12cm cuffs, 2x2.5cm ...
Manufactured by:Newman Medical based inArvada, COLORADO (USA)
The ABI-Q comes with a computer with simpleABI Software, PVR, 2x10cm cuffs, inflation bulb, carry bag, user manual, quick reference guides. ...
Manufactured by:Newman Medical based inArvada, COLORADO (USA)
Our most advanced Cuff-Link vascular system with remote control and automated cuff-selection. The 600CL performs all lower extremity physiologic arterial exams: (i) Single level protocols (ii) Multi-level protocols (iii) Exercise protocols. The ABI-600CL comes with simpleABI Software, 8 port Cuff-Link™ with remote, D8 Doppler probe, DPPG ...
Manufactured by:Newman Medical based inArvada, COLORADO (USA)
An advanced Cuff-Link vascular system with remote control and automated cuff selection. The 500CL performs lower extremity physiologic arterial exams including: (i) Single level protocols (ii) Multi-level protocols. The ABI-500CL comes with simpleABI Software, 8 port Cuff-Link™ with remote, D8 Doppler probe, DPPG probe, 2x10cm cuffs, 4x12cm ...
Manufactured by:RTI Group AB based inMölndal, SWEDEN
The GE Vascular Holder is designed for accurate positioning of the Piranha and Barracuda Dose Probes on the GE Vascular ...
Manufactured by:Rex Medical, L.P. based inConshohocken, PENNSYLVANIA (USA)
Closer™ Vascular Sealing System is not commercially available. Large Bore Closer™ Vascular Sealing System has not been approved for clinical investigation or commercial distribution in the U.S. Closer™/Large Bore Closer™ is a trademark of Rex Medical, patents ...
Manufactured by:Unetixs Vascular, Inc. based inWarwick, RHODE ISLAND (USA)
To make your diagnostic testing procedures more efficient, use the Unetixs infrared temperature instrument with any UnetixsMultiLab® vascular diagnostic system when conducting non-contact Raynaud studies. The non-contact method saves the time required for attaching sensors when other modalities are used. Digit temperatures can be accurately obtained in about 1/3 second per site. With other ...
Manufactured by:Universal Diagnostic Solutions based inVista, CALIFORNIA (USA)
The Vista ABI system was designed for practices that want to begin performing ABI exams now and also have the option of later addressing accessories like the PPG probe and software. The accompanying compact printer rapidly prints two ankle waveforms along with the ABI ...
Manufactured by:CP Medical based inNorcross, GEORGIA (US) (USA)
Superior microbial barrier. Helps to prevent key-part touch contamination. Allows to visually assess and confirm that a proper flush has been ...
Manufactured by:Unetixs Vascular, Inc. based inWarwick, RHODE ISLAND (USA)
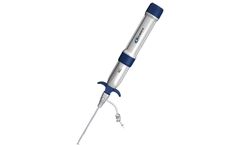
Unetixs 5 MHz and 8 MHz continuous wave (CW) TruDop® Doppler probes achieve the highest levels of accuracy, durability and comfort. Try them and you will be impressed by their dynamic range and superior sensitivity. Moreover, the drop-tested durability of Unetixs composite probe tips relieves the stress and replacement expense associated with accidentally damaged conventional probes. ...
Manufactured by:Unetixs Vascular, Inc. based inWarwick, RHODE ISLAND (USA)
Speed up your exams with Unetixs versatile PPG (photoplethysmography) digit clips and sensors! No application of tape or Velcro is required for most PPG exams and you can easily remove the sensors from their securely docked position in the clip for cleaning and replacement or for separate use in thoracic-outlet-syndrome and other tests. It is easy to obtain accurate waveforms from even the ...
Manufactured by:Canadian Hospital Specialties Limited (CHS) based inOakville, ONTARIO (CANADA)
The Smartdop® XT6 is the ideal vascular ABI and PPG toe pressure Doppler. With 6 inflation ports, a simple one-button start, and dual side cuff inflation, the Smartdop® XT6 can perform a complete bilateral ABI study in minutes. Integrated pulse volume and PPG (photoplethysmography) probes enable a wide variety of arterial and venous studies for accurate assessment of vascular disease. ...
Manufactured by:Remington Medical Inc. based inAlpharetta, GEORGIA (US) (USA)
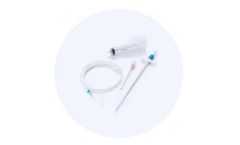
Introducer set used for placement of a .038-inch diameter guide wire into the vascular ...
Manufactured by:Thompson Surgical Instruments based inTraverse City, MICHIGAN (USA)
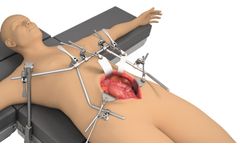
Achieve unsurpassed general and vascular exposure with the Thompson General / Vascular System. The system contains a selection of abdominal blades ideal for general abdominal and vascular procedures, especially aortic repair. Sterile mount rail clamp and independent frame arms allow for versatile setups and when combined with S-Lock Quick Angle handles, the system gives surgeons control over each ...
Manufactured by:PFM Medical, Inc. based inCarlsbad, CALIFORNIA (USA)
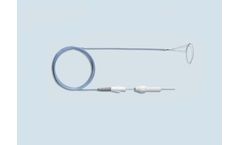
The Multi-Snare Device is a patented, highly efficient multiple loop retrieval snare with a dual-plane design to grasp objects from numerous angles. Designed with various reliable pull-force strengths, the kink resistant nitinol dual loop system grants the ability to grasp, manipulate or assist from any angle or vessel sidewall. The Multi-Snare MAX Device has been designed with the same ...
Manufactured by:Cardival Medical, Inc. based inSanta Clara, CALIFORNIA (USA)
VASCADE MVP eliminates the need for manual compression and reduces time to ambulation by 64%. Get patients up and moving hours earlier, with significantly less discomfort. The only FDA-approved closure device for use following cardiac ...
Manufactured by:Integer Holdings Corporation based inPlano, TENNESSEE (USA)
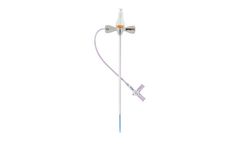
Introducing the Adelante® SafeSheath® II Hemostatic Peel Away Introducer System for Vascular Access. This generation peel away introducer features the latest lubricated hemostatic valve membrane that provides low insertion forces during procedures. It also includes a side port with a 3-way stopcock that provides a convenient means of aspirating and flushing the ...
Manufactured by:Cordis based inMiami Lakes, FLORIDA (USA)
Intended for use in the intravascular introduction of interventional or diagnostic devices for coronary or peripheral vascular ...
Manufactured by:Reprise Biomedical based inPlymouth, MINNESOTA (USA)
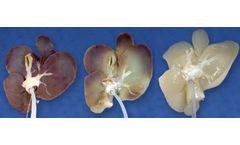
Our proprietary perfusion decellularization process involves cannulating the main vasculature of the organ and perfusing a mild detergent solution through the native blood vessels to result in a preserved native scaffold containing the appropriate strength and microenvironment for cellular integration after it is implanted in the patient’s ...
Manufactured by:Universal Diagnostic Solutions based inVista, CALIFORNIA (USA)
This full-featured vascular assessment system is ideally suited to perform segmental exams. The Vista AVS offers three modalities for obtaining systolic pressures and waveforms and is designed to accommodate both standard and custom ...