- Home
- Equipment
- north america
- whole dried blood samples
Show results for
Refine by
Whole Dried Blood Samples Equipment Supplied In North America
78 equipment items found
Manufactured by:Tasso, Inc. based inSeattle, WASHINGTON (USA)
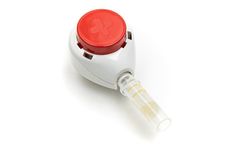
The Tasso-M20 device delivers whole dried blood samples from the patient to the lab. It can be used for PK (pharmacokinetic) monitoring in patients enrolled in clinical trials. Subjects can successfully collect volumetrically precise samples regardless of access to trial sites. ...
Manufactured by:Chrono-log Corporation based inHavertown, PENNSYLVANIA (USA)
Each vial contains 2.5mg of lyophilized adenosine diphosphate. Performs 500 to 1,000 tests in PRP or Whole Blood ...
Manufactured by:Chrono-log Corporation based inHavertown, PENNSYLVANIA (USA)
Each vial contains a minimum of 10 units of lyophilized Thrombin from human plasma. Performs 10 to 20 tests in PRP or Whole Blood ...
Manufactured by:Chrono-log Corporation based inHavertown, PENNSYLVANIA (USA)
Each vial contains a minimum of 10mg of Arachidonic Acid, purity better than 99%. Included is a vial containing 100mg bovine albumin, fraction V powder, 96% to 99% purity. Performs 70 to 140 tests in PRP or Whole Blood ...
Manufactured by:Chrono-log Corporation based inHavertown, PENNSYLVANIA (USA)
Each vial contains 62.5mg of stabilized freeze dried Ristocetin. Performs 62 to 125 tests in PRP or Whole Blood ...
Manufactured by:BUHLMANN Diagnostics Corp based inAmherst, NEW HAMPSHIRE (USA)
The Flow CAST®(Cellular Allergy Stimulation Test) is intended for the determination of basophil activation in whole blood samples by flow cytometry. Our unique marker combination – CCR3 and CD63 – combines the simple gating properties for basophil detection by CCR3 with the solid application of the proven activation marker CD63 in one. Thanks to its easy protocol and smart marker ...
Manufactured by:Tasso, Inc. based inSeattle, WASHINGTON (USA)
The Tasso+ device collects whole liquid blood samples that are ready to be used for a variety of downstream applications. Multiple standard collection tubes are compatible. Users can successfully collect blood samples at home without direct interaction at a lab or with a mobile ...
Manufactured by:Chrono-log Corporation based inHavertown, PENNSYLVANIA (USA)
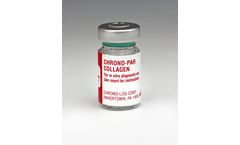
Each vial contains 1mg of native Collagen fibrils (type I) from equine tendons suspended in isotonic glucose solution of pH 2.7. Performs 500 to 1,000 tests in PRP or Whole Blood ...
Manufactured by:Chrono-log Corporation based inHavertown, PENNSYLVANIA (USA)
Reagent for measurement of ATP release. Each vial contains 0.2 mg luciferin, 22,000 units d-luciferase plus magnesium sulfate, human serum albumin, stabilizers and buffer. Kit includes 4 vials of CHRONO- LUME plus a vial of lyophilized adenosine 5' triphosphate for use as an ATP Standard. Performs 50 to 100 tests in PRP or Whole Blood ...
Manufactured by:Tasso, Inc. based inSeattle, WASHINGTON (USA)
The Tasso-SST device delivers whole liquid blood samples prepared without anticoagulation, allowing immediate separation of serum for antibody and other testing. Adults and children can successfully collect blood samples at home without direct interaction at a lab or with a mobile phlebotomist. These devices are currently available for investigational use only ...
Manufactured by:HORIBA Medical based inOberursel, GERMANY
The reference in fully automated smearing and staining: High throughput: 120 slides per hour; Continuous loading; Whole blood 75µl sampling; Automatic ...
Manufactured by:NOWDiagnostics Inc. based inSpringdale, ARKANSAS (USA)
The ADEXUSDx Troponin I Test is an immunochromatographic assay used for the qualitative detection of human cardiac Troponin I above an established cut-off in human whole blood, plasma, or serum samples as an aid for healthcare professionals in the diagnosis of myocardial ...
Manufactured by:More Diagnostics Inc. based inLos Osos, CALIFORNIA (USA)
More Diagnostics’ Cyclosporine C2 Control has assayed values for Siemens Dimension® and Vista®; Thermo Fisher Cedia® and Siemens Syva EMIT 2000® and is also appropriate for other automated immunoassay systems that correlate with chromatographic ...
Manufactured by:Able Diagnostics, Inc. based inSan Diego, CALIFORNIA (USA)
900 Memories with date and time, and 7, 14, and 30-day averaging; 5 Test reminders to help form a test routine; Ketone Warning and Hypo Warning reduce risks caused by extreme glucose values; Auto-Shutoff in 2 minutes after the last ...
Manufactured by:i-SENS, Inc. based inSeocho-gu, SOUTH KOREA
Accurate results in 5 seconds with 0.5 μL sample size. High, reliable accuracy & precision. View test results with large ...
Manufactured by:Able Diagnostics, Inc. based inSan Diego, CALIFORNIA (USA)
Code Calibration (auto-coding). Confirms if Sample Size is Sufficient. Eliminates the HCT (hematocrit interference). Eliminates Temperature Interference. Checks for Possible Damage to the Test Strip. Checks for Humidity Exposure. Confirms if the Sample is Blood or Control ...
Manufactured by:Links Medical Products Inc. based inIrvine, CALIFORNIA (USA)
The FORA GD20 provides safe, accurate results and is intended for multi-patient use in a long-term care ...
Manufactured by:HemoSonics, LLC based inDurham, NORTH CAROLINA (USA)
The QPlus Cartridge provides semi-quantitative indications of the coagulation state of a 3.2% citrated venous whole blood sample. For use in perioperative patients age 18 years and older to assess possible hypocoagulable and hypercoagulable conditions. ...
Manufactured by:NOWDiagnostics Inc. based inSpringdale, ARKANSAS (USA)
The ADEXUSDx® Methanol Dip Test is an enzymatic assay used for the qualitative detection of methanol above an established cut-off in human whole blood, plasma, or serum samples in clinical laboratory settings as an aid for healthcare professionals in the diagnosis of methanol ...
Manufactured by:Links Medical Products Inc. based inIrvine, CALIFORNIA (USA)
Glucose monitoring has never been easier… Bigger Buttons in a compact device. Easy to use for elderly people. ...