- Home
- Equipment
- north america
- wound healing process
Show results for
Refine by
Wound Healing Process Equipment Supplied In North America
10 equipment items found
Manufactured by:Canadian Hospital Specialties Limited (CHS) based inOakville, ONTARIO (CANADA)
ActiGraft System, a patented proprietary technology was developed by RedDress, a privately held company based in Israel. Their goal is to develop more effective, natural and economically viable treatments for Chronic Wounds. The first of its kind technology enables health care providers to produce in real time – an in vitro blood clot from a patient’s whole blood. ...
Manufactured by:CP Medical based inNorcross, GEORGIA (US) (USA)
SutureSeal™ is the only wound sealant that provides up to 14 days of wound protection with just 1 application. Use on: staples, sutures, other wound closures and open ...
Manufactured by:Sciton, Inc. based inPalo Alto, CALIFORNIA (USA)
Sciton’s Fractionated Erbium Laser Goes Deep Without the Downtime. Sciton’s fractionated erbium laser is the ideal treatment for scar revision, deep resurfacing, and healthy skin ...
Manufactured by:Phoenix Wound Matrix by RenovoDerm based inDublin, OHIO (USA)
The Phoenix Wound Matrix is a 3-dimensional wound care device designed to provide a temporary microenvironment to support wound healing. The device is engineered to promote regeneration and remodeling of native tissue in the defect space/wound bed. The Phoenix Wound Matrix is made of polymers ...
Manufactured by:Femasys Inc. based inSuwanee, GEORGIA (US) (USA)
FemBloc features our proprietary delivery platform, which places balloon technology close to the opening of both fallopian tubes. This in-office approach is designed to eliminate the risks of incisions, anesthesia, and hormones. As a nonsurgical procedure that is implant free, FemBloc delivers a biopolymer that is expected to expel within 3 months. Confirmation of procedure success can be ...
Manufactured by:Avery Dennison Medical based inChicago,, ILLINOIS (USA)
The Absorbent Wound Dressing portfolio is indicated for the management of lightly to moderately exuding chronic and acute wounds. The range is designed to promote a moist wound environment, which can support healing. The portfolio includes products designed for partial and full thickness pressure sores and leg ulcers as well as superficial wounds, skin tears and postoperative ...
Manufactured by:Lohmann & Rauscher GmbH & Co. KG (L&R) based inRengsdorf, GERMANY
The recommended dressing change interval is normally 2–4 days. Depending on the type of wound and its healing progress, the dressing can be left on the wound for a shorter or longer period (up to a maximum of 7 ...
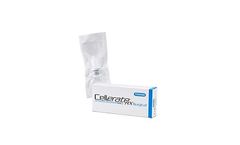
Manufactured by:Sanara MedTech Inc. based inFort Worth, TEXAS (USA)
CellerateRX® Surgical Powder is hydrolyzed collagen that provides a moist environment for surgical sites and wounds. CellerateRX® Surgical Powder is composed of hydrolyzed fragments of Type I Bovine Collagen that are approximately 1/100th the size of native ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Lacto-N-neotetraose (LNnT) is an endogenous metabolite. Lacto-N-neotetraose can inhibit TNF-α induced IL-8 secretion in immature epithelial cells. Lacto-N-neotetraose has anti-inflammatory avtivity, and can improve the wound ...
Manufactured by:OxyBand Technologies Inc. based inTiburon, CALIFORNIA (USA)
OxyBand Technologies, Inc. is the first company to package pure oxygen into a discrete traditional wound dressing, using a directionally permeable gas emitting reservoir. OxyBand's patented technology delivers the therapeutic value of oxygen to accelerate wound healing and fight ...