- Home
- Equipment
- south korea
- biocompatibie
Refine by
Biocompatibie Equipment Supplied In South Korea
16 equipment items found
Manufactured by:TaeWoong Medical based inGyeonggi-do, SOUTH KOREA
There are 4 different types for managing tracheobronchial obstruction. ...
Manufactured by:Alfa Medical based inSeocho-Gu, SOUTH KOREA
HA Concentration : 20 mg/ml. Micro Needle Size (included) : 30G 13mm. Duration : 1 year. Shelf Life : 2.5 years. Application : Crow’s feet, Earlobe, Perioral Wrinkles, Glabella lines, Forehead ...
Manufactured by:Alfa Medical based inSeocho-Gu, SOUTH KOREA
HA Concentration : 20 mg/ml. Micro Needle Size (included) : 27G 13mm. Duration : 1 year. Shelf Life : 2.5 years. Application : Cheek, Chin, Fore-head, Nose, Laugh lines, Nasolabial lines, Lip Contouring, Glabella ...
Manufactured by:Alfa Medical based inSeocho-Gu, SOUTH KOREA
HA Concentration : 20 mg/ml. Micro Needle Size (included) : 31G 13mm. Duration : 2 months. Shelf Life : 2.5 years. Application : Whole ...
Manufactured by:Alfa Medical based inSeocho-Gu, SOUTH KOREA
HA Concentration : 20 mg/ml. Micro Needle Size (included) : 27G 13mm. Duration : 1 year. Shelf Life : 2.5 years. Application : Nasolabial lines, Nose, Cheek, Chin, Fore-head, Laugh lines, Lip ...
Manufactured by:Hurev Co., Ltd. based inWonju-si, SOUTH KOREA
StiMus Silicone Electrode is a silicone electrode for low frequency stimulation manufactured in Hurev Co.,Ltd. It is safe because silicone containing biocompatible data is used as the raw material and unlike existing low frequency pad. it can be used almost semi permanently by replacing consumable items[in case HydroGel Patch is ...
Manufactured by:Alfa Medical based inSeocho-Gu, SOUTH KOREA
It is largely effective in facial lifting and wrinkle removal through self-stimulation of cell. There is no side effect or worries about removal operation because it will be fully dissolved in the body. In addition there is no interruption in daily life even right after the procedure. This is biodegradable, biocompatible, safe and dissolves in the body. ...
Manufactured by:Meta Biomed Co,. Ltd. based inCheongju-si, SOUTH KOREA
Application: Protect pulp of the tooth. Lining under all filling material. Protection when applying the total etch technique. ...
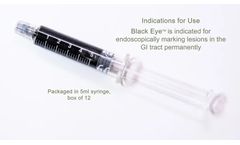
Manufactured by:Black Eye Endoscopic Marker based inGunpo-si,, SOUTH KOREA
Black Eye™ is a sterile, non-pyrogenic, purified carbon formulation designed to be used as an endoscopic marker for marking polyps and other lesions in the gastrointestinal tract. Black Eye™ is supplied in a ready-to-use, pre-filled syringe containing 5ml of Black Eye™ marker, manufactured by The Standard Co. Ltd. Shelf life is 2 years. The safe choice for your patients: ...
Manufactured by:Jeil Medical Corporation based inGuro-gu, SOUTH KOREA
Temporary Anchorage Devices(TADs) - Orthodontic Mini Screws ...
Manufactured by:Hurev Co., Ltd. based inWonju-si, SOUTH KOREA
It is a disposable electrocardiogram measurement electrode with general monitoring, stress test, restring type and radiography environment model depending on the purpose of use.Skin irritation was minimized by applying raw materials containing biocompatible ...
Manufactured by:Nano Intelligent Biomedical Engineering Corporations (NIBEC) Co., Ltd. based inJincheon-gun, SOUTH KOREA
This product is a 'medical device'. Please carefully read 'Precautions' and ‘Instruction' before use. OssGen-X15 is a compound of bone graft material (OCS-B) / (OCS-H) and bioactive peptides. OssGen-X15 consists of bioactive peptides with hydroxyapatite-binding peptides. OssGen-X15 can stably bind to the bone graft material (OCS-B or OCS-H) via hydroxyapatite-binding peptides. The bone ...
Manufactured by:Nano Intelligent Biomedical Engineering Corporations (NIBEC) Co., Ltd. based inJincheon-gun, SOUTH KOREA
Regenomer is Sponge-like absorbable and porous collagen matrix). Made from purified type I atelocollagen derived from porcine skin sources in South Korea). Physically crosslinked). Biocompatible). Recommended resorption time is 4 ...
Manufactured by:Nano Intelligent Biomedical Engineering Corporations (NIBEC) Co., Ltd. based inJincheon-gun, SOUTH KOREA
OCS-B Xenomatrix bone graft material is bone mineral derived from bovine bone. Inflammatory organic components have been completely removed by rigorous manufacturing processes, and the structure of OCS-B Xenomatrix has a high resemblance to that of human bone. Helping osteoblasts facilitate smooth bone matrix synthesis and bone calcification, OCS-B Xenomatrix significantly promotes reconstruction ...
Manufactured by:Nano Intelligent Biomedical Engineering Corporations (NIBEC) Co., Ltd. based inJincheon-gun, SOUTH KOREA
“This product is a 'medical device'. Please carefully read 'Precautions' and ‘Instruction' before use.”. Manufactured by removing organic components and purifying only inorganic bone mineral, OCS-H has a low rate of inflammation and immune rejection. It is a biocompatible bone graft material that is made from equine bone, of which structure is almost the same as human bone, so ...
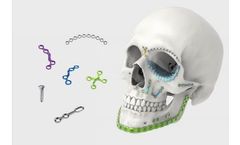
Manufactured by:Jeil Medical Corporation based inGuro-gu, SOUTH KOREA
The LeForte System is a titanium bone plating system to stabilize bone fractures or osteotomies on cranial, oral, maxillofacial sites in Oral and Maxillofacial Surgery, Plastic and Neuro ...