- Home
- Equipment
- south korea
- clinical case studies
Show results for
Refine by
Clinical Case Studies Equipment Supplied In South Korea
12 equipment items found
Manufactured by:IBEX Medical Systems Co., Ltd. based inGangwon-do,, SOUTH KOREA
Sweat-reducing effects in clinical test results / US FDA Approved / Non-invasive Medical ...
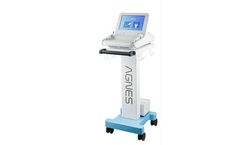
Manufactured by:AGNES Medical Co., Ltd. based inSeongnam-si, SOUTH KOREA
AGNES-S emits customized level of RF energy to target in dermis through the Micro-Insulated Needle. Its unique, but safe square waveform of RF energy minimizes damages on adjacent tissues and the Micro-Insulated Needle ensures the precise control of RF energy to coagulate sebaceous glands for Acne and Blackhead and eccrine glands for Syringoma ...
by:Vivozon Inc. based inYongin-si, SOUTH KOREA
On July 29, 2020, Vivozon applied for a patent for a second pipeline, VVZ-2471, and is currently conducting non-clinical toxicity studies. VVZ-2471 is a new drug candidate for treating chronic pain including neuropathic pain. Recently, the prevention and treatment potential of drug (morphine) addiction, anxiety, and depression have also been demonstrated through non-clinical studies. ...
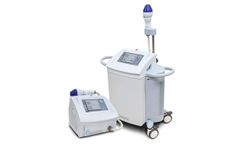
Manufactured by:AGNES Medical Co., Ltd. based inSeongnam-si, SOUTH KOREA
Over the years of ceaseless efforts to provide innovative and stable solution to physicians and patients, AGNES has become one of the most dynamic and innovative developers of the RF energy-based aesthetic and medical treatment system. Developing our own system and treatment protocols, our mission is to provide the satisfaction to physicians and the opportunity of success and growth to our ...
Manufactured by:Medispec based inGaithersburg, MARYLAND (USA)
The E3000 ESWL System is an Extracorporeal Shock Wave Lithotripter that effectively treats renal & ureter calculi. With more than a 1000 systems sold worldwide to the world’s leading medical institutions, the E3000 system offers the ideal combination between largest focal zone in the industry and bar pressure, resulting in superior results in clinical studies with high stone-free rate ...
Manufactured by:ExoAtlet based inLuxembourg, LUXEMBOURG
ExoAtlet I is an exoskeleton for research and early mobility for patients with locomotor disorders. Since 2017 we have been supporting more than 25 clinical research projects with such nosologies as spinal cord injury, cerebral palsy, stroke, multiple sclerosis, traumatic brain injury and athropalthy in different clinics worldwide. Since 2019 several American and European projects have started ...
by:Agaram Technologies based inChennai, INDIA
Interfacer middleware for Invitro diagnostic device interfacing. Interfacer is a configurable middleware for interfacing clinical diagnostic ...
Manufactured by:Hue Light Co., Ltd. based inGwangmyeong-si, SOUTH KOREA
The Whole-Body Photobiomodulation Treatment Device by Huelight is a state-of-the-art system engineered to deliver therapeutic light across the entire body. Utilizing over 40,000 medical-grade LEDs, this device emits red and near-infrared wavelengths designed to penetrate deeply into tissues. The primary function is to stimulate cellular energy production via mitochondria, enhancing recovery and ...
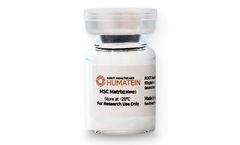
Manufactured by:ROKIT Healthcare based inGeumcheon-gu, SOUTH KOREA
A cell culture biomaterial supplement consisting of full native human ECM (Extracellular Matrix), useful for in vitro 4D bioprinting and cell culturing as well as clinical translational ...
Manufactured by:Medispec based inGaithersburg, MARYLAND (USA)
Erectile Dysfunction Shock Wave Therapy (EDSWT) is an innovative approach to vasculogenic ED. Medispec’s ED1000 is a medical device that uses advanced acoustics technology based on electrohydraulic power. This technology has proven to be effective in cardiology, with recent success in reversible ischemic tissues of the heart. EDSWT utilizes low-intensity extracorporeal shock waves, focusing ...
Manufactured by:LISCure Biosciences Inc. based inSeongnam-si, SOUTH KOREA
The LMT (LISCure Microbiology-based Technology) platform is an innovative tool to keep discovering and analyzing microbial and genome-based drug candidates. LMT (LISCure Microbiology-based Technology) Platform Consists of the Therapeutic Screening Platform, Microbial Optimization Platform, and Microbiome Database ...
Manufactured by:Markes based inBridgend, UNITED KINGDOM
Markes’ BioVOC-2™ breath sampler is a lightweight and simple-to-use device designed for non-invasive biological exposure monitoring of VOCs. Using a design pioneered by the UK Health & Safety Executive, BioVOC-2 collects the last 129 mL of expired breath for transfer to a sorbent tube, and subsequent analysis by thermal desorption. BioVOC-2 is for research use only. Not for use in ...