- Home
- Equipment
- south korea
- diagnostics technology for diagnostics medical
Show results for
Refine by
Diagnostics Technology For Diagnostics Medical Equipment Supplied In South Korea
7 equipment items found
Manufactured by:OPTOLANE Technologies Inc. based inSeongnam-si, SOUTH KOREA
LOAA Analyzer(model name : LOAA-M) is real-time PCR instrument which is an in vitro diagnostic (IVD) medical device used to amplify specific genes (DNA or RNA) designed for use with cartridges. PCR data is automatically analyzed by the dedicated software installed in the device and results are delivered to users through on-screen display or exported to external memory for further ...
Manufactured by:TPC Mechatronics based inSeo-gu, SOUTH KOREA
ISO 13485 (Medical Devices: Quality Management Systems), 1 Light source, 1 Detection (No Crosstalk). 4 Channel Multiplex(Covid19 + General Flu Simultaneous Testing). Advanced modular optic module. ...
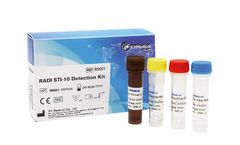
Manufactured by:KH Medical based inPyeongtaek-si, SOUTH KOREA
The RADI STI-10 Detection KIT is an in vitro diagnostic medical device, based on Real-time RT-PCR technology to detect the common 10 sexually transmitted pathogen ...
Manufactured by:OPTOLANE Technologies Inc. based inSeongnam-si, SOUTH KOREA
LOAA Analyzer is Real-time PCR instrument which is an in vitro diagnostic (IVD) medical device used to amplify specific genes (DNA or RNA) designed for use with cartridges. PCR reaction data is automatically analyzed by the dedicated software installed in the device and results are delivered to users through on-screen display or export to external memory for further processing. ...
Manufactured by:Bilix Co., Ltd. based inYongin, SOUTH KOREA
An ultrasound imaging device is one of the most widely used medical diagnostic technologies, with its excellent mobility and accessibility. It is the safest and fastest diagnostic technique compared to others such as magnetic resonance imaging(MRI) and computed tomography(CT). Despite such advantages, one of the limitations of ...
Manufactured by:LG Chem, Ltd. based inSeoul, SOUTH KOREA
A reagent for in vitro diagnostic devices that detect semi-quantitatively allergen-specific IgE antibodies by immunoblotting in human serum or plasma treated with heparin, citrate, or CPDA-1 as an anticoagulant. (Results can be read by AdvanSure™ AlloView 1.0 only) ...
Manufactured by:TPC Mechatronics based inSeo-gu, SOUTH KOREA
ISO 13485(Medical device quality control) certification. Standard Deep Well Plate(Open Platform) application. Reasonable Price(70% compared to competitors), High performance. ...