Refine by
Drug Release Delivery Equipment Supplied In Sweden
11 equipment items found
Manufactured by:SHL Medical AG based inZug, SWITZERLAND
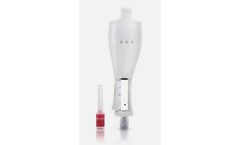
The development of the Precision Pen Injector (PPI) is a story of uncompromising dedication to meeting industry challenges. A Swedish company specializing in solutions for aesthetics and corrective treatment sought to introduce an autoinjector for its skin booster – a highly viscous hyaluronic acid formulation of several hundred centipoise. The company needed a device that could deliver ...
Manufactured by:SHL Medical AG based inZug, SWITZERLAND
SHL’s dedication to designing patient-centric products is evident in our very first injector, the PenInject ...
Manufactured by:SHL Medical AG based inZug, SWITZERLAND
Inheriting the classic Molly’s compact design and simplicity of use, this second-generation Molly autoinjector is designed with a new ergonomic body and a flange-shaped anti-roll cap for an easier handling experience. Built on a modular platform technology, Molly is designed to meet the evolving requirements of future autoinjectors, such as introducing unique designs for branding and user ...
Manufactured by:SHL Medical AG based inZug, SWITZERLAND
The establishment of the Molly platform signifies SHL’s commitment to further addressing the varying needs of our customers and their patients. Introduced in 2010, Molly is SHL’s first preconfigured autoinjector designed to help pharmaceutical companies reduce initial investments and expedite development ...
Manufactured by:SHL Medical AG based inZug, SWITZERLAND
2006 marked the commercial launch of one of the world’s first modern autoinjectors – SHL’s Disposable Autoinjector, more commonly known as DAI. The first DAI was built for a multinational biopharmaceutical company headquartered in the US. It is a button-activated, 3-step disposable device for 1 mL pre-filled syringes. We were tasked to design an injection device that could ...
Manufactured by:Recipharm AB based inStockholm, SWEDEN
Recipharm has extensive experience in the injectables area and supplies small volume parenterals in ampoules and vials, as well as in cartridges. State of the art leak detection and automatic inspection equipment is used and Recipharm’s full service offering is complete with secondary packaging and temperature controlled ...
Manufactured by:Micrel Medical Devices SA based inAthens, GREECE
The MP Daily+ is a simple and reliable system that provides infusion therapy for a wide range of applications. It is truly ambulatory with a robust design, trouble-free set up and accurate delivery in mm/24hr. A clear informative display giving easy alarm identification. The pump is driven by a dual micro-processor that safeguards smooth operation in any drug delivery applications. ...
Manufactured by:Micrel Medical Devices SA based inAthens, GREECE
The MP mlh+ Multi Syringe is a simple and reliable syringe driver using 10 to 50 ml standard syringes and that provides infusion therapy for a wide range of applications. It is truly ambulatory with a robust design, trouble-free set up and accurate delivery in ml/hr with bolus option. A clear informative display giving easy alarm identification with a dual micro-processor construction gives the ...
Manufactured by:Micrel Medical Devices SA based inAthens, GREECE
The MP 101+ is a simple and reliable syringe driver that provides infusion therapy for a wide range of applications. It is truly ambulatory with a robust design, trouble-free set up and accurate delivery in mm/hr. A clear informative display gives easy alarm identification. The pump is driven by a dual micro-processor that safeguards smooth operation in any drug delivery ...
Manufactured by:Mölnlycke Health Care AB based inGothenburg, SWEDEN
BARRIER EasyWarm should be used by or under the supervision of a qualified health care professional. BARRIER EasyWarm is intended for perioperative use in ...
Manufactured by:Vivesto AB based inSolna, SWEDEN
Many intravenously delivered Active Pharmaceutical Ingredients (APIs) are insoluble or poorly soluble in water. This can be a major hurdle in pharmaceutical development and may cause promising drugs to fail during the development process and may limit the application of approved drugs because of poor solubility. According to some estimates, between 70 and 90% of drugs in the development pipeline ...