Show results for
Refine by
Pharmaceutical Ingredients Equipment Supplied In Sweden
6 equipment items found
Manufactured by:Perstorp Holding AB based inMalmö, SWEDEN
Valeric Acid Pure is a high purity carboxylic acid. Valeric Acid Pure is developed especially for high demanding applications in API (Active Pharmaceutical Ingredient) production (for example acid chlorides - Valeroyl ...
Manufactured by:Vivesto AB based inSolna, SWEDEN
XR-18 is a research effort based on the XR-17 technology platform intended to provide enhanced properties that improve clinical formulations and applications of active pharmaceutical ingredients (APIs) for cancer treatment. This effort has recently generated promising data including: Addition of components to existing XR-17 formulation improving certain ...
Manufactured by:Diamyd Medical AB based inStockholm, SWEDEN
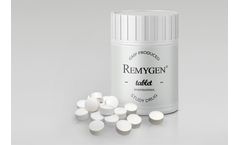
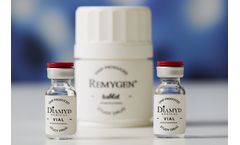
Remygen® is a controlled release tablet consisting of the active pharmaceutical ingredient GABA. The GABA substance is chemically synthesized in a well-controlled GMP process together with a global contract manufacturer. The manufacturing process is scalable and pediatric formulations with the same properties can be designed. ...
Manufactured by:Diamyd Medical AB based inStockholm, SWEDEN
Diamyd® is a suspension for injection containing the active pharmaceutical ingredient GAD65 mixed with the vaccine adjuvant Alhydrogel (alum). The human recombinant protein GAD65 is manufactured in insect cells in a well-controlled GMP process. The manufacturing process is developed for late phase clinical use. ...
Manufactured by:Vivesto AB based inSolna, SWEDEN
Many intravenously delivered Active Pharmaceutical Ingredients (APIs) are insoluble or poorly soluble in water. This can be a major hurdle in pharmaceutical development and may cause promising drugs to fail during the development process and may limit the application of approved drugs because of poor solubility. ...
Manufactured by:ErtelAlsop based inKingston, NEW YORK (USA)
The pharmaceutical and biotech markets require a high level of functional consistency from filtration products. To provide a higher level of confidence for these customers, ErtelAlsop drafted its Drug Master File and submitted it to the FDA Center for Drug Evaluation and Research. ErtelAlsop manufactures all filter pads to very high standards, and the Drug Master File provides the documentation ...