- Home
- Equipment
- switzerland
- catheters
Refine by
Catheters Equipment Supplied In Switzerland
28 equipment items found
Manufactured by:Cordis based inMiami Lakes, FLORIDA (USA)
Intended for use in the intravascular introduction of interventional or diagnostic devices for coronary or peripheral vascular ...
Manufactured by:ICU Medical, Inc. based inSan Clemente, CALIFORNIA (USA)
Designed to quickly establish non-operative pleural drainage for the relief of life-threatening chest conditions such as tension pneumothorax. Ideally suited for insertion by blunt dissection, as advocated by modern trauma and emergency management ...
Manufactured by:Neotec Medical based inSingapore, SINGAPORE
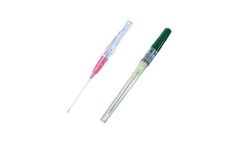
Features: Protection against needlestick injuries. No special cannulation technique required. Self-activating Tip cover technology, no user activation required. Safety mechanism cannot be bypassed. Backcut bevel reduces pain of insertion. Advanced Tip design for smoother transition. Transparent Flashback Chamber with Hydrophobic Filter for consistent flashback. Available in PTFE, FEP and ...
Manufactured by:Sahajanand Medical Technologies Ltd. based inSurat, INDIA
Low diameter growth between nominal and rated burst pressure. Low entry & crossing profile. Predictable balloon COMPLIANCE - to allow precise diameter sizing. Short INFLATION & DEFLATION time. Kink resistant ...
Manufactured by:Sahajanand Medical Technologies Ltd. based inSurat, INDIA
Low tip entry profile. Low crossing profile. Quick Inflation & Deflation: SMT's proprietary technology ensures quick deflation (lower deflation time results in reduced ischemic complications). Kink resistant ...
Manufactured by:Neotec Medical based inSingapore, SINGAPORE
Features: Protection against needlestick injuries. No special cannulation technique required. Self-activating Tip cover technology, no user activation required. Safety mechanism cannot be bypassed. Backcut bevel reduces pain of insertion. Advanced Tip design for smoother transition. Transparent Flashback Chamber with Hydrophobic Filter for consistent flashback. Available in PTFE, FEP and ...
Manufactured by:Cordis based inMiami Lakes, FLORIDA (USA)
Intended for percutaneous entry and guidance of catheters, the Cordis EMERALD Diagnostic Guidewire complements our diagnostic catheter and catheter sheath introducer lines. Performance, endurance and safety are built into each EMERALD Guidewire with solid tensile strength to minimize the likelihood of stretching or fracturing. ...
Manufactured by:Neotec Medical based inSingapore, SINGAPORE
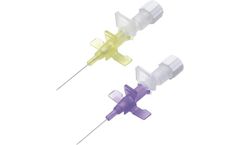
Protection against needlestick injuries. Same cannulation technique as in conventional IV Catheter. Self-activating Tip cover technology, no user activation required. Safety mechanism cannot be bypassed. Backcut bevel reduces pain of cannulation. Advanced Catheter Tip design for smoother transition. Transparent Flashback Chamber with Hydrophobic Filter for ...
Manufactured by:Neotec Medical based inSingapore, SINGAPORE
Features: Color-coded catheter hub body as per EN ISO – 10555. USP standards Class VI compliance, thin walled Catheters with PTFE, PUR, FEP or ETFE Clear, Radio-opaque is also available. Gently tapered catheter tip with Customized Automated Tipping Technology (CATT) for lower penetration forces and optimal trim distance to provide smooth ...
Manufactured by:Neotec Medical based inSingapore, SINGAPORE
Features: Protection against needlestick injuries. Same cannulation technique as in conventional IV Catheter. Self-activating Tip cover technology, no user activation required. Safety mechanism cannot be bypassed. Backcut bevel reduces pain of cannulation. Advanced Catheter Tip design for smoother transition. Transparent Flashback Chamber with Hydrophobic Filter ...
Manufactured by:Neotec Medical based inSingapore, SINGAPORE
Colour coded body & wings for size identification. Siliconised, ultra-sharp back-cut needle for painless venipuncture. Kink resistant and biocompatible catheter with smooth surface for easy penetration. Advance tip design provides smooth transition from needle to catheter. ...
Manufactured by:Neotec Medical based inSingapore, SINGAPORE
HYDROPHOBIC FILTER allows consistent flashback. Siliconised, ultra-sharp back-cut needle for painless venipuncture. Kink resistant and biocompatible catheter with smooth surface for easy penetration. Advance tip design provides smooth transition from needle to catheter. Sterile, disposable and pyrogen free. ...
Manufactured by:Cordis based inMiami Lakes, FLORIDA (USA)
The Cordis AVANTI+ Introducer is a pioneer of catheter sheath introducer technology. For use in arterial and venous procedures requiring percutaneous introduction of Intravascular devices. ...
Manufactured by:atHeart Medical based inBaar, SWITZERLAND
Some may close on their own early in life, while others require an intervention. Today, the vast majority are performed through minimally invasive catheter-based interventions. They consist in implanting an atrial septal occluder through a transcatheter procedure to close the defect. Current ASD occluder devices have dense metal frames that permanently clamp the septum. The ...
Manufactured by:Elektrisola Medical Technologies based inBoscawen, NEW HAMPSHIRE (USA)
Specialized constructions using a variety of conductor and insulation options are available. Medical Grade Thermocouple wire is typically used in catheter applications and supplied to customer specified lengths or packaging requirements. ...
Manufactured by:Elektrisola Medical Technologies based inBoscawen, NEW HAMPSHIRE (USA)
Specialized constructions using a variety of conductor and insulation options are available. Medical Grade Thermocouple wire is typically used in catheter applications and supplied to customer specified lengths or packaging requirements. ...
Manufactured by:ABCDx based inGeneva, SWITZERLAND
LVOs alone lead to 95% of all disabilities caused by strokes. Today LVOs can be treated by mechanical thrombectomy, the mechanical removal of the thrombus operated with a ...
Manufactured by:Spirecut AG based inMuttenz, SWITZERLAND
The instrument's cutting edge ensures safe and precise release of target structures, aided by its echogenic lateral flanges for enhanced visibility under sonography. Another notable design feature is its 1.5mm diameter rod, which is compatible with standard 14 Gauge IV catheter access. Intended exclusively for use by skilled surgeons under sterile conditions, the device bypasses ...
Manufactured by:InnoMedicus based inCham, SWITZERLAND
The patented system allows an effective heat exchange with a constant temperature of 43 ±0.5 °C, constant pressure and volume flow during the entire treatment period and, for the benefit of the patient, the use of a slim 16 Fr catheter. Successful in clinical use since 2011, it is safe for patients and medical staff and has a comparable patient tolerability to bladder ...
Manufactured by:Spirecut AG based inMuttenz, SWITZERLAND
The Trigger Finger Sono-Instrument provides a minimally invasive solution for treating trigger fingers through percutaneous release of the annular pulley, particularly the A1 pulley. Packaged as a single-use sterile kit, it includes a probe that assists in tissue dissection and confirms the surgical release's completeness. The procedure is performed under ultrasound guidance with local ...