- Home
- Equipment
- switzerland
- class iii ce certified medical devices
Refine by
Class Iii Ce Certified Medical Devices Equipment Supplied In Switzerland
3 equipment items found
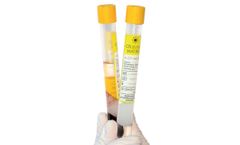
Manufactured by:Regen Lab SA based inVaud, SWITZERLAND
Cellular Matrix® tubes allow the preparation of autologous platelet-rich plasma (RegenPRP®) combined with a non-crosslinked hyaluronic acid (HA) in a closed-circuit system. CM-PRP-HA has an excellent safety profile in clinical practice.1,3,4. Cellular Matrix® technology combines the complementary clinical effects of RegenPRP® and hyaluronic acid (HA), giving better and ...
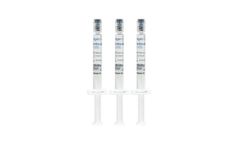
Manufactured by:Regen Lab SA based inVaud, SWITZERLAND
ArthroVisc 40 is a non-cross-linked hyaluronic acid (2 %) obtained from bacterial fermentation. This product is sterile and non-pyrogenic. It is packaged in 2 ml syringes of hyaluronic acid (20 mg/ml, 1500 KDa). ArthroVisc 40 is available in three syringes of 2 ml. It is for single use and designed to be used with sterile needles (25G or 27G), not supplied in the kit. ...
Manufactured by:Micro Crystal AG based inGrenchen, SWITZERLAND
The CC8A-T1A Medical is a high frequency SMT Quartz Crystal Unit built with an AT-Cut Crystal Resonator. Manufactured in accordance with the highest quality production technologies, it operates under vaccum in a Helium impervious full ceramic package. CC8A-T1A Medical is dedicated for specific use in Class III implantable medical devices and benefits from long-term reliability which is of utmost ...