- Home
- Equipment
- united kingdom
- clinical and operational data
Show results for
Refine by
Clinical And Operational Data Equipment Supplied In United Kingdom
15 equipment items found
Manufactured by:Wright Medical Group N.V. based inMemphis, TENNESSEE (USA)
Key Benefits: A solution for hands disabled by osteoarthritis and post-traumatic arthritis. Dorsal hinge design shows no sign of failure after 10 million flexion cycles*. Confidence in over 40 years of clinical use. *Data on file at ...
Manufactured by:CN Bio based inCambridge, UNITED KINGDOM
NASH-in-a-box contains everything required to recreate our industry-validated human in vitro Non-alcoholic steatohepatitis (NASH) model in your own ...
Manufactured by:Futura Medical based inGuildford, UNITED KINGDOM
TIB200 is a topical 10% ibuprofen gel for pain relief that utilises Futura's DermaSys® technology. DermaSys® enables ibuprofen to be delivered rapidly and efficiently through the skin. Local application and rapid, efficient absorption translate into a fast onset of action, and the potential for twice-daily ...
Manufactured by:Futura Medical based inGuildford, UNITED KINGDOM
Scientific advisory meeting held with MHRA confirming the need of a Phase 3 study to support the improved skin permeation including potential superior efficacy claims. Exploring the feasibility of a clinical study to satisfy the Phase 3 requirements for both UK and US approval. Development currently on ...
Manufactured by:Brandon Medical Co Ltd based inLeeds, UNITED KINGDOM
iTCP is an “intelligent” digital, programmable, connected device with integral computers and communications. They can be used as stand-alone items, integrated with 3rd party equipment or connected together as part of our Brandon Equipment Package (BEP). ...
Manufactured by:Benevolent based inLondon, UNITED KINGDOM
Better target discovery and compound design in Ulcerative Colitis. What is UC?: Ulcerative colitis (UC) is a chronic, lifelong disease that causes inflammation and ulceration of the inner lining of the colon and rectum, accompanied by debilitating symptoms. Our work: There is no cure for UC, current treatments have side effects and don’t work on all patients. Our work focuses on developing ...
Manufactured by:Sequana Medical NV based inSint-Denijs Westrem, BELGIUM
Direct Sodium Removal (DSR®) therapy is Sequana Medical’s novel and proprietary approach under development for the management of fluid overload due to heart failure. The sodium concentration in patients with fluid overload is in balance but there is too much sodium and too much fluid in the body. Sequana Medical’s approach is to remove excess sodium in patients with ...
Manufactured by:Benevolent based inLondon, UNITED KINGDOM
The Benevolent Platform™ is our powerful computational R&D platform. At its core sits our Knowledge Graph, which captures the interconnectivity of all relevant available data and scientific literature. Our suite of exploratory and predictive AI tools allow scientists to interrogate the data and disease networks within the graph, ask biological questions, surface novel ...
Manufactured by:Microbiotica Limited based inLittle Chesterford, UNITED KINGDOM
Microbiotica accesses high quality large-scale clinical datasets in order to identify microbiome-based biomarkers of drug response, drug side-effects or disease progression. The company has accessed the clinical data from academic/clinical collaborations (University of Adelaide, Cambridge University Hospitals/Cancer Research UK) or from pharma partners ...
Manufactured by:Vitalograph Inc based inLenexa, KANSAS (USA)
The COMPACT Medical Workstation forms the foundation of our PFT solutions and is a validated medical device for clinic use, with a user friendly interface and integral spirometer. The device hosts questionnaires and centralizes data from a range of pulmonary function tests and other medical ...
Manufactured by:Destiny Pharma plc based inBrighton, UNITED KINGDOM
The XF drug platform has a novel, ultra-rapid mechanism that reduces the chance of bacteria becoming resistant to its action. Destiny Pharma’s XF platform has advantages over traditional antibiotics. ...
Manufactured by:Acurable Limited based inLondon, UNITED KINGDOM
AcuPebble RE100 is a wearable device that fundamentally improves the measurement of respiratory biosignals. It provides more accurate data and enables the continuous monitoring of patients at home in clinical trials and post-market ...
Manufactured by:Veryan Medical Ltd. based inHorsham, UNITED KINGDOM
The Challenge: Peripheral Arterial Disease. People are living longer and with an increase in the incidence of obesity, diabetes and other contributing factors, claudication may cause substantial tissue changes and, in some scenarios, can lead to amputation and even death. The peripheral stent market is forecast to grow to $2 billion by 2024 driven by technological advances, an ageing population ...
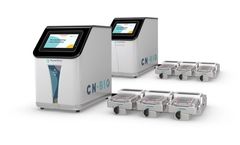
Manufactured by:CN Bio based inCambridge, UNITED KINGDOM
Generate human-specific pre-clinical safety and efficacy data with pioneering PhysioMimix single- and multi-organ-on-a-chip ...
Manufactured by:Skeletal Dynamics, LLC based inMiami, FLORIDA (USA)
Designed to effectively fix the proximal tip of the olecranon and prevent avulsion of the triceps ...