- Home
- Equipment
- united kingdom
- clinical case study
Show results for
Refine by
Clinical Case Study Equipment Supplied In United Kingdom
71 equipment items found
Manufactured by:Menssana Research Inc. based inEdgewater, NEW JERSEY (USA)
Every year in the U.S.A., 99,000 men and 78,000 women develop lung cancer, and only 14% of them are still alive five years later. Fortunately, early detection and treatment can reduce the number of deaths. Clinical studies at Menssana Research, Inc. have shown that breath biomarkers accurately identify lung cancer, and this has been confirmed in a blinded replicated ...
Manufactured by:Ben’s Natural Health based inLondon, UNITED KINGDOM
Prostate Healer is a unique Ayurvedic blend updated for the modern age, embodying scientifically refined ancient wisdom. Cold-pressed and macerated herbal ingredients are formulated to relieve bacterial prostatitis and help maintain optimal prostate ...
Manufactured by:Creative Diagnostics based inShirley, NEW YORK (USA)
With heart disease being such a huge problem across the globe, Cardiac research is at the pinnacle of modern day biological studies. Our range of Cardiac markers Antibodies continues to grow, offering new products designed to advance your clinical research projects and aid in identification and treatment of acute cardiac conditions in a diagnostic environment. We aim to provide high quality tools ...
Manufactured by:Precision For Medicine, Inc. based inFrederick, MARYLAND (USA)
Clinical and research-grade leukopaks - mobilized or non-mobilized from healthy and diseased subjects, including oncology donors. ...
Manufactured by:Diagnosys LLC based inLowell, MASSACHUSETTS (USA)
The Dark Adaptometry module uses the ColorDome™ or ColorFlash™ stimulator to perform a full or partial field dark adaptation to accurately measure retinal parameters such as cone sensitivity, rod sensitivity and the rod-cone break point. The dark adaptometry test may be run for single or dual eye measurements or a combination. Protocols include the Marmor Test as well as a wide array ...
Manufactured by:Alnylam Pharmaceuticals, Inc. based inCambridge, MASSACHUSETTS (USA)
AMVUTTRA™ (vutrisiran) is a prescription medicine given once every 3 months by a healthcare professional to treat the polyneuropathy caused by an illness called hereditary ATTR (hATTR) amyloidosis in adults. In an 18-month clinical study, AMVUTTRA-treated patients showed significant improvement in nerve function and quality of life at 9 months and continued to improve throughout the ...
Manufactured by:Inspiration Healthcare Group based inCroydon, UNITED KINGDOM
The Inspire rPAP™ is a revolutionary, 2-piece non-invasive system for the initial stabilisation and resuscitation of infants. It’s innovative, patented design combines the ability to administer inflation breaths with all the clinical benefits of the gold standard fluidic flip nCPAP technology. Following birth, the first moments are crucial in defining the respiratory management ...
Manufactured by:Wolfmet based inManchester, UNITED KINGDOM
Wolfmet 3D, developed by M&I Materials Ltd, leverages a sophisticated selective laser melting (SLM) technique for additive manufacturing. This process involves the use of a high-powered laser to meticulously fuse layers of pure tungsten powder. The result is the creation of highly intricate and precise components such as collimators and radiation shields used in medical imaging systems like ...
Manufactured by:MR Solutions based inGuildford, UNITED KINGDOM
MR SOLUTIONS presents its 10th generation MRI spectrometer console, the EVO2, designed for controlling and operating both preclinical and clinical MR imaging systems. This advanced console builds on the proven success of the EVO spectrometer. With over 2000 units installed globally, MR SOLUTIONS is a leading independent developer and supplier of MRI spectrometers to OEMs and individual users. The ...
Manufactured by:Futura Medical based inGuildford, UNITED KINGDOM
TIB200 is a topical 10% ibuprofen gel for pain relief that utilises Futura's DermaSys® technology. DermaSys® enables ibuprofen to be delivered rapidly and efficiently through the skin. Local application and rapid, efficient absorption translate into a fast onset of action, and the potential for twice-daily ...
Manufactured by:Erchonia Lasers Ltd. based inWallingford, UNITED KINGDOM
The FX 635® Laser is Erchonia’s flagship device for pain relief. It is the only LLLT machine to be FDA Market Cleared for chronic heel pain arising from plantar fasciitis, and chronic low back pain of musculoskeletal ...
Manufactured by:ReGen Therapeutics Limited based inLondon, UNITED KINGDOM
The sedative zolpidem (marketed by Sanofi-Aventis under the trade names Stilnox™ and Ambien™) has been used for the last twenty years as a hypnotic/sedative by millions of people. It is now off patent and an alternative use has now been discovered which is owned by ...
Manufactured by:BTL Aesthetics based inNewcastle, UNITED KINGDOM
EMSCULPT NEO is the first and only non-invasive body shaping procedure that combines 2 energies for simultaneous fat elimination and muscle building in a 30-minute ...
Manufactured by:Futura Medical based inGuildford, UNITED KINGDOM
Scientific advisory meeting held with MHRA confirming the need of a Phase 3 study to support the improved skin permeation including potential superior efficacy claims. Exploring the feasibility of a clinical study to satisfy the Phase 3 requirements for both UK and US approval. Development currently on ...
Manufactured by:Erchonia Lasers Ltd. based inWallingford, UNITED KINGDOM
Erchonia’s non-thermal lasers are the only Low-Level Laser Therapy (LLLT) devices to be FDA Market Cleared for treating toenail and nail fungus (onychomycosis). The efficacy of our machines has been proven by multiple (level 1) double blind, randomised, placebo-controlled, and multi-site clinical studies – the most credible research in the market today. The treatments are short, ...
Manufactured by:Menssana Research Inc. based inEdgewater, NEW JERSEY (USA)
It takes 300 screening mammograms to discover one woman with breast cancer. A breath test might improve this very low yield. Researchers have known for many years that women with breast cancer have abnormal volatile organic compounds (VOCs) in their breath. Clinical studies at Menssana Research have shown that these breath VOCs can accurately identify women with early stage breast cancer or an ...
Manufactured by:Destiny Pharma plc based inBrighton, UNITED KINGDOM
Background to the NTCD-M3 Clostridioides difficile Programme. NTCD-M3 was developed by GI infection physician, Professor Dale Gerding, who is a world-leading specialist in C. difficile, with more than 400 peer-reviewed journal publications, book chapters and review articles in the area. NTCD-M3 has successfully completed Phase 1 and Phase 2b trials with NTCD-M3. The Phase 1 study demonstrated a ...
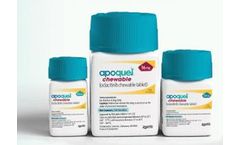
Manufactured by:Zoetis Services LLC based inParsippany, NEW JERSEY (USA)
Apoquel is the #1 prescribed oral medicine for fast, effective relief of allergic itch and inflammation due to atopic and allergic dermatitis. Apoquel is available as a Tablet and a Chewable Tablet. Chewable Tablet was formulated in response to pet owner preferences for an easy-to-dose option. ...
Manufactured by:Treos Bio Limited based inLondon, UNITED KINGDOM
Off-the-shelf products contain 6-12 shared tumor-specific antigen derived peptides optimized for a population. They are personalized with a candidate companion diagnostic test (CDx). In silico trials predicted that these peptides induce exceptionally broad T cell responses against at least 3 tumor-specific antigens in high proportion of patients, without need of biopsy. Phase 1 clinical study ...
Manufactured by:Sequana Medical NV based inSint-Denijs Westrem, BELGIUM
Direct Sodium Removal (DSR®) therapy is Sequana Medical’s novel and proprietary approach under development for the management of fluid overload due to heart failure. The sodium concentration in patients with fluid overload is in balance but there is too much sodium and too much fluid in the body. Sequana Medical’s approach is to remove excess sodium in patients with ...