- Home
- Equipment
- united kingdom
- colonizing
Refine by
Colonizing Equipment Supplied In United Kingdom
17 equipment items found
Manufactured by:Creative Bioarray based inShirley, NEW YORK (USA)
Human colon cells are isolated from human normal colon section and are ideal research models for malignant and non-malignant colorectum diseases. The cells are characterized by immunofluorescence with antibodies specific to CK18, CK19 and GAPDH. Human colon cells are cryopreserved at passage 7 and delivered frozen. T25 flasks is required for cell ...
Manufactured by:Sigma-Aldrich based inBellefonte, PENNSYLVANIA (USA)
3dGRO® Human Colon Organoid Expansion Medium is a complete ready-to-use serum-free medium for the expansion and long-term culture of human colonic organoids. The medium has been validated for use with 3dGRO® Human iPSC Derived Colon Organoids (Cat. No. SCC300). Colon organoids propagated in the medium express ...
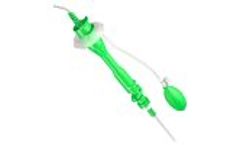
Manufactured by:APR Medtech Ltd based inOxon, UNITED KINGDOM
The severity of the transit problem is quantifiable by the transit test and the result may be an important variable; it may facilitate the decision on further diagnostic procedures, it effects the choice of therapy and long-term prognosis. Segmental and total colonic transit time is calculated according to the distribution of the markers in the different segments of the bowel. ...
Manufactured by:Benevolent based inLondon, UNITED KINGDOM
: Ulcerative colitis (UC) is a chronic, lifelong disease that causes inflammation and ulceration of the inner lining of the colon and rectum, accompanied by debilitating symptoms. Our work: There is no cure for UC, current treatments have side effects and don’t work on all patients. Our work focuses on developing safer and more effective oral small molecule treatments. ...
Manufactured by:Quay Pharma based inFlintshire, UNITED KINGDOM
They are used to enhance systemic delivery by directing the release of the API to specific areas of the small intestine or colon, and targeted delivery in this way may be important for several reasons. Active ingredients that are labile or that may irritate the stomach can be coated to pass through the stomach intact. For the treatment of local diseases such as ulcerative ...
Manufactured by:ICU Medical, Inc. based inSan Clemente, CALIFORNIA (USA)
When you choose ICU Medical needlefree IV sets and connectors with patented Clave™ technology, you get an effective barrier against bacterial transfer and colonization designed to help reduce the risk of bloodstream infections. And since the same clinical protocol is used with all patient populations, you can standardize on a single connector technology wherever care is ...
Manufactured by:ColoWrap, LLC based inDurham, UNITED KINGDOM
ColoWrap is specifically designed to prevent looping. Looping occurs in up to 90% of colonoscopies and is the reason why manual pressure and patient repositioning is required. ColoWrap is the first device specifically designed to mitigate looping - minimizing the need for manual pressure and repositioning and keeping staff and patients ...
Manufactured by:Quay Pharma based inFlintshire, UNITED KINGDOM
Microparticles are spherical particles between 0.1μm and 100μm in diameter. They fall into two broad groups. Microspheres are homogenous mixtures of polymers and active ingredients, and microcapsules have a core of one substance surrounded by a shell of a different ...
Manufactured by:Destiny Pharma plc based inBrighton, UNITED KINGDOM
Background to the NTCD-M3 Clostridioides difficile Programme. NTCD-M3 was developed by GI infection physician, Professor Dale Gerding, who is a world-leading specialist in C. difficile, with more than 400 peer-reviewed journal publications, book chapters and review articles in the area. NTCD-M3 has successfully completed Phase 1 and Phase 2b trials with NTCD-M3. The Phase 1 study demonstrated a ...
by:Exact Sciences UK, Ltd. based inLondon, UNITED KINGDOM
Every cancer is unique and affects each diagnosed person differently based on the individual biology of his or her disease. To address this diversity and make cancer care smarter at every stage, our Oncotype DX genomic tests and services portfolio delivers clinically relevant genomic intelligence that reveals the individual biology of a patient's ...
Manufactured by:MD Diagnostics Ltd based inChatham, UNITED KINGDOM
The H2 Check is suitable for all age groups of patients. Its principle use is for the simple diagnosis of Lactose, fructose or sucrose Intolerance. Using a special re-breathing technique, patients are required to simply tidal breath into the H2 Checkfor immediate results in parts per million ...
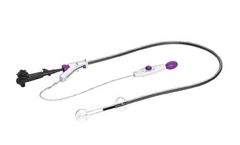
Manufactured by:Lumendi Ltd. based inMaidenhead, UNITED KINGDOM
Pioneering Endosurgery: we are blazing a new trail of healing and discovery. We are pursuing innovation because patients deserve ...
Manufactured by:Stericom Ltd. Part of the P3 Medical Ltd based inBristol, UNITED KINGDOM
ColpoWave is the world’s first colpotomizer with a 20mm oncology margin. It can be rotated back and forth for clear visual landmarks and physical safety margins at all stages of the ...
Manufactured by:Stericom Ltd. Part of the P3 Medical Ltd based inBristol, UNITED KINGDOM
CerviGrip is designed to work with ColpoWave colpotomizer to enable cervical fixation and uterine manipulation by either the surgical assistant, or directly by the surgeon if the SurgiAssist Uterine Positioner is deployed during the ...
Manufactured by:Stericom Ltd. Part of the P3 Medical Ltd based inBristol, UNITED KINGDOM
The SurgiAssist Uterine Positioner is a robust, reusable mechanical device which anchors to the operating table to provide a stable pelvic platform throughout surgery. Human beings get tired – SurgiAssist ...
Manufactured by:Lumendi Ltd. based inMaidenhead, UNITED KINGDOM
EZ Glide is an innovative, proprietary, hydrophilic coating applied to DiLumen’s inner sheath. Activated by simply water or saline, EZ Glide reduces the need for messy accessory lubricants. The result is an easier, faster and cleaner preparation, with a marked improvement in endoscopic stability, visibility, and control. ...
Manufactured by:Scancell based inOxford, UNITED KINGDOM
The Modi-2 vaccine exploits a second post-translational modification, stimulating the production of CD4 T cells using tumour-associated peptide epitopes in which the lysine residues are converted to homocitrulline. This change occurs via a process known as carbamylation, leading to a change in molecular charge which, in turn, alters antigenic properties and can result in the generation of unique ...