- Home
- Equipment
- united kingdom
- drug master file document production
Show results for
Refine by
Drug Master File Document Production Equipment Supplied In United Kingdom
14 equipment items found
Manufactured by:Copley Scientific Ltd based inColwick, UNITED KINGDOM
Copley offers a range of testing equipment for additional qualification and validation of orally inhaled and nasal drug products (OINDPs), including assessments of generic drug formulations, inhaled dissolution testing and improving IVIVCs. View Our Inhaler Testing Brochure at: ...
Manufactured by:Evox Therapeutics Limited based inOxford, UNITED KINGDOM
Phenylketonuria, or PKU, is an autosomal recessive inborn error of phenylalanine metabolism resulting in a deficiency of the hepatic enzyme phenylalanine hydroxylase ...
Manufactured by:Blueberry Therapeutics based inCheshire, UNITED KINGDOM
Onychomycosis is a disease caused by fungal infection of the nail and is an extremely common disorder worldwide. Onychomycosis is very common; prevalence rates are as high as 23% in Europe, 20% in East Asia and 14% in North America, and are expected to increase further. It becomes more common with increased age and in some patients with conditions such as diabetes and poor peripheral circulation. ...
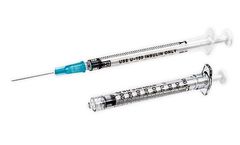
Manufactured by:Becton Dickinson and Company (BD) based inFranklin Lakes, NEW JERSEY (USA)
Our 1-mL BD Luer-Lok™ insulin syringe—available with either a detachable needle or a permanently attached needle—support many clinical uses and crucially prevent needle disengagement, medication leakage, and spray or tubing disconnect. We also offer a 1-mL insulin syringe with the BD Luer-Lok tip or the U-100 slip tip. These insulin syringes are all available at many drug ...
Manufactured by:SureScreen Diagnostics Ltd. based inDerby, UNITED KINGDOM
Since the first use of fingerprint identification in 1858, we've known that the combination of whorls, arches and loops that make up the pattern of our fingerprint is unique to each ...
Manufactured by:SureScreen Diagnostics Ltd. based inDerby, UNITED KINGDOM
Drug metabolites begin appearing in urine a couple of hours after a substance is taken. These metabolites can be detectable in a urine sample for several days and up to 30 days after use. Whether using a dip or cup screening test, urine presents the widest range of parameters for drug screening, and is often the first matrix to feature new parameters. With best practice procedures in place, urine ...
Manufactured by:Quay Pharma based inFlintshire, UNITED KINGDOM
Lipophilic systems have proven effectiveness in improving bioavailability by helping drugs to overcome problems like poor gastric solubilisation, degradation and elimination due to first-pass metabolism in the liver, other types of pre-systemic metabolism, and drug transport and efflux effects. At Quay Pharma, we are highly experienced in identifying the most suitable lipid excipients for the ...
Manufactured by:Powder Systems Limited (PSL) based inEstuary Banks, UNITED KINGDOM
The LabMSR system is a GLP piece of equipment that has been developed to enable global drug manufacturers to generally replicate, at R&D scale, some of the process benefits of PSL’s full scale production MicroSphere Refiners (MSR). Due to their characteristics and properties, polymeric microspheres have traditionally been known to be difficult to process for manufacturers – ...
Manufactured by:Catalent, Inc based inSomerset, NEW JERSEY (USA)
The biologics market is experiencing high growth. However, the path to clinic for biologics is complex, requiring a substantial amount of time and resources to ensure success. Sponsor companies are looking for an “all of the above” solution, where they don’t have to trade speed for risk while managing multiple development partners. ...
Manufactured by:Envair Technology based inHeywood, UNITED KINGDOM
Envair Technology's CDC F Negative Pressure Isolator is designed to safeguard pharmacy and laboratory professionals from hazardous exposure to cytotoxic drugs while ensuring microbiological contamination prevention. It provides versatility with options for two or four glove configurations and customizable working areas, enhancing operational ease. These isolators are equipped with additional ...
Manufactured by:Sepha Limited based inDundonald, UNITED KINGDOM
The Sepha EZ Blister is a table top blister packaging machine, ideal for packaging tablets, capsules, ampoules, sachets, pouches and other ...
Manufactured by:SureScreen Diagnostics Ltd. based inDerby, UNITED KINGDOM
The biological content of oral fluid is directly relational to blood plasma. Therefore, if we detect drug metabolite in oral fluid, those metabolites are still present in the blood stream, indicating that the substance has recently been active in the body; so oral fluid is the choice matrix for ‘for-cause’ testing, but can also be used for random and pre-employment situations where ...
Manufactured by:Powder Systems Limited (PSL) based inEstuary Banks, UNITED KINGDOM
The MSR™ MicroSphere Refiner is a cGMP piece of equipment that allows drug developers and manufacturers to complete a wide range of aseptic processes with their microspheres at various scales. It is a one-of-its-kind solution has been developed and refined over decades following a Quality-by-Design (QbD) approach, taking into account the characteristics of microspheres and process ...
Manufactured by:Agilent Technologies, Inc. based inSanta Clara, CALIFORNIA (USA)
BioTek Cytation 7 cell imaging multimode reader combines automated digital upright and inverted widefield microscopy with conventional multimode microplate reading. Cytation 7 includes continually variable bandpass monochromators for versatility and performance for general multimode plate reader applications. The inverted microscopy module provides sample visualization and the upright microscopy ...