- Home
- Equipment
- united kingdom
- medical device directive
Refine by
Applications
- Parasym - Non-Invasive Neuromodulation Device for Vagus Nerve
- Parasym - Non-Invasive Neuromodulation Device for Vagus Nerve Stimulation (VNS / tVNS)
- Parasym - Non-Invasive Neuromodulation Device for Stimulating the Vagus Parasympathetic Activation
- Parasym - Non-Invasive Neuromodulation Device for Mood Enhancement / Neural Modulation with tVNS
- Medical Device for Clinicians
- Medical Device for Patients
Medical Device Directive Equipment Supplied In United Kingdom
16 equipment items found
Manufactured by:Inotec AMD based inCambridge, UNITED KINGDOM
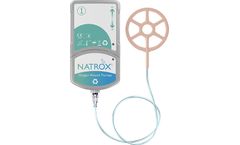
NATROX Oxygen Wound Therapy System is a class II medical device licensed under Medical Device Directive 93/42/EEC. The system consists of two main elements; the NATROX Oxygen Generator and the NATROX Oxygen Delivery System. ...
Manufactured by:Luxfer MEL Technologies based inManchester, UNITED KINGDOM
Our development and manufacture is the ISO 13485 quality system, this is a requirement for future medical device regulations. Our dedicated facility offers high quality material and process control, this lowers customers risk. We can support programmes from kg to tonne; scalability and capability to support programmes through to success. Luxfer are technical experts in the field with experience ...
Manufactured by:Vernacare based inBolton, UNITED KINGDOM
Single-use components in one sterile pack to be used to assist in cleaning and dressing ...
Manufactured by:Parasym Ltd. based inLondon, UNITED KINGDOM
The Parasym device delivers non-invasive Neuromodulation to harness beneficial therapeutic mechanisms of the vagus ...
Manufactured by:Vernacare based inBolton, UNITED KINGDOM
Single use Double Ended Amalgam Condenser, designed to compact or condense a restorative material into a prepared dental cavity. Supplied in an easy to open, sterile ...
Manufactured by:AgPlus Diagnostics Ltd based inSharnbrook, UNITED KINGDOM
To work in conjunction with our proven sensitive assay development service, we are proud to have launched The Agilis Reader. This pairing delivers the market requirement of a rapid, ultra-sensitive, fully quantitative Point of Care test for use across a wide range of clinical and non-clinical applications. The multi-application capability of the new AgPlus platform will see it meeting many ...
Manufactured by:Vernacare based inBolton, UNITED KINGDOM
Single use Adson Tissue Forceps, used for fine surgical procedures to hold delicate or superficial tissues. Also used to tie sutures at the end of the procedure and hold dressings. Manufactured from medical grade surgical stainless steel. Supplied in an easy to open, sterile ...
Manufactured by:Terumo Aortic based inRenfrewshire, UNITED KINGDOM
TREO provides a wide range of endovascular device configurations to address the abdominal anatomy of each individual ...
Manufactured by:Vitalograph Inc based inLenexa, KANSAS (USA)
The Vitalograph 6MWT solution includes a tablet running the 6MWT protocol, SPO2 monitor and kit to mark the ...
Manufactured by:Terumo Aortic based inRenfrewshire, UNITED KINGDOM
RELAY® endovascular devices are designed to help you achieve maximum precision and control in the thoracic ...
Manufactured by:Connect Medical Systems Limited (CMS) based inLancashire, UNITED KINGDOM
High quality, safe and easy to install: This quick and easy-to-install medical gas outlet is a cost-effective solution for anyone looking for a high-quality, British-made product from a reputable UK manufacturer with decades of experience in designing and producing medical gas pipeline systems. For hospital and laboratory settings: The terminal unit is suitable for piped medical gas installations ...
Manufactured by:Tristel Solutions Ltd. based inSnailwell, UNITED KINGDOM
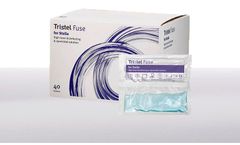
Tristel Fuse for Stella is a single-use disinfectant solution designed specifically for use in Stella for the high-level disinfection of semi-critical medical devices. Tristel Fuse for Stella incorporates two separate compartments that contain 50ml Tristel Base solution (citric acid) and 50ml Tristel Activator solution (sodium chlorite). When mixed upon bursting the sachet, Tristel’s ...
Manufactured by:International Scientific Supplies Ltd, Part of the STH Plastics Group based inBradford, UNITED KINGDOM
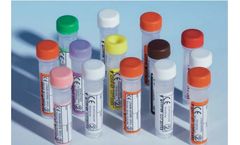
Standard Cap; Here is what to expect when you buy these tubes: Screw cap tubes for 0.5 – 1.3ml of blood. The tubes are made with the highest quality polypropylene. These long narrow tubes aid storage and “harvest”. We are able to provide these tubes with a range of additives or ...
Manufactured by:Tristel Solutions Ltd. based inSnailwell, UNITED KINGDOM
Combining the simplicity of manual disinfection with a semi-automated washer disinfector, the Stella System offers quick, mobile, and economic reprocessing of single-lumened and non-lumened, heat-sensitive medical devices. With a high-level disinfection cycle of five minutes, or when cleaning is included a total cycle time of ten minutes, the Stella System enables medical devices to be ...
Manufactured by:Acurable Limited based inLondon, UNITED KINGDOM
Clinically validated: AcuPebble SA100 automated sleep apnea test was validated in a powered clinical trial at the NHS Royal Free London hospital. Validated sleep study result equivalent to ambulatory gold-standard (multi-channel polygraphy followed by manual specialist interpretation). Validated result based on AHI and ODI for both 3% and 4% desaturation criteria with high accuracy (94% PPV, 98% ...
Manufactured by:Tristel Solutions Ltd. based inSnailwell, UNITED KINGDOM
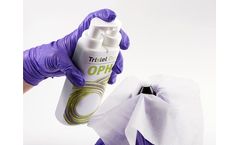
Tristel Duo OPH is an effective, fast and compliant high-level disinfectant for ophthalmic and optometry medical devices. Within ophthalmology and optometry, clinicians are required to ensure the highest level of infection control is adhered to. However, eye care is often viewed as a low-risk area where a low-level disinfectant will be suitable, resulting in inadequate device disinfection. ...