- Home
- Equipment
- united kingdom
- medical devices
Refine by
Applications
- Parasym - Non-Invasive Neuromodulation Device for Vagus Nerve
- Parasym - Non-Invasive Neuromodulation Device for Vagus Nerve Stimulation (VNS / tVNS)
- Parasym - Non-Invasive Neuromodulation Device for Stimulating the Vagus Parasympathetic Activation
- Parasym - Non-Invasive Neuromodulation Device for Mood Enhancement / Neural Modulation with tVNS
- Neuromuscular Electrostimulation Device for VTE Prevention – Obstetrics - Hospital Applications
- Neuromuscular Electrostimulation Device for Post-Operative Oedema Prevention - Hospital Applications
- Neuromuscular Electrostimulation Device for VTE Prevention - Acute Stroke- Hospital Applications
- Solutions for Glaucoma and Intraocular Pressure (IOP)
- Medical Device for Clinicians
- Medical Device for Patients
- Neuromuscular Electrostimulation Device for Pre-operative Oedema Reduction - Ankle Fracture - Hospital Applications
- Wound Therapy Applications Device for Mixed Aetiology Leg Ulcers
- Wound Therapy Applications Device for Venous Leg Ulcers
- Neuromuscular Electrostimulation Device for Post-operative Oedema Reduction - Hip Replacement - Hospital Applications
- Neuromuscular Electrostimulation Device for VTE Prevention – NICE Guidance- Hospital Applications
Medical Devices Equipment Supplied In United Kingdom
119 equipment items found
Manufactured by:Plasma Surgical, Inc. based inRoswell, GEORGIA (US) (USA)
PlasmaJet delivers effective control to surgeons: Allows enhanced visualization, opening, and dissection of tissue planes. Controlled tissue effect enables use on or around sensitive structures, leading to more complete disease removal. Less than 0.3 mm depth of thermal effect on key structures such as bowel, ovary, and fallopian tubes. Less than 0.5 mm lateral thermal spread. Sealing of small ...
Manufactured by:Bio Molecular Systems based inUpper Coomera, AUSTRALIA
The world’s first magnetic induction cycler is now a registered medical device with CE-IVD and TGA approval. Mic IVD is manufactured under an ISO 13485:2016 Quality Management System. ...
Manufactured by:Jamak based inWeatherford, TEXAS (USA)
Silicone medical devices. Silicone Rubber Elastomer Industrial Consumer Design Sourcing Logistics ...
Manufactured by:Terumo Aortic based inRenfrewshire, UNITED KINGDOM
Gelsoft™ Plus is a surgical solution that enables you to achieve anatomical equilibrium in the ...
Manufactured by:Terumo Aortic based inRenfrewshire, UNITED KINGDOM
Gelweave™ delivers a wide range of solutions for the thoracic ...
Manufactured by:Rapid Biosensor Systems Ltd based inCambridge, UNITED KINGDOM
Dr Ruth McNerney is a senior lecturer at the London School of Hygiene and Tropical Medicine, Britain's national school of public health and an internationally-recognised centre of excellence in tropical medicine ...
Manufactured by:Tristel Solutions Ltd. based inSnailwell, UNITED KINGDOM
Tristel Protect provides short-term storage and transportation for reusable medical devices. Tristel Protect is designed to transport or store clean devices for up to 72-hours. For emergency situations such as patient intubation, Tristel Protect keeps your device clean and ready to use. Tristel Protect can also be used to ...
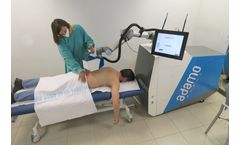
Manufactured by:Adamo Robot based inMadrid, SPAIN
The world’s first collaborative robot that treats MSDs with compressed air. For Rehabilitation Centers and Hospitals – Clinics or Sport Clubs. ADAMO is a robotic medical device capable of aiding in the diagnosis of back pain injuries and delivering contactless treatment using temperature-controlled pressurized air. ADAMO can help reduce patient ...
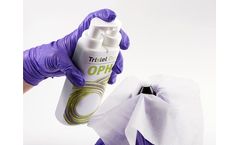
Manufactured by:Tristel Solutions Ltd. based inSnailwell, UNITED KINGDOM
Duo Wipes are the perfect partner for Tristel Duo OPH and Tristel Duo ULT. Duo Wipes are dry wipes made of a soft, durable, low-linting fabric that will not leave a buildup of lint on medical devices. Each wipe is low absorbing to ensure the maximum amount of foam is distributed onto the medical ...
by:Accruent based inAustin, TEXAS (USA)
Manage attributes of all network connected medical devices. Conduct risk assessments, reconcile MDS2 data and other security attributes against inventory, and remediate potential risks and vulnerabilities. Automate workflows unique to your ...
Manufactured by:Sepha Limited based inDundonald, UNITED KINGDOM
The Sepha EZ MedPak is a compact, lab scale medical device packaging machine that has been designed specifically for the medical device market where sterility maintenance and barrier protection against microbial penetration is ...
Manufactured by:Tristel Solutions Ltd. based inSnailwell, UNITED KINGDOM
Tristel Duo OPH is an effective, fast and compliant high-level disinfectant for ophthalmic and optometry medical devices. Within ophthalmology and optometry, clinicians are required to ensure the highest level of infection control is adhered to. However, eye care is often viewed as a low-risk area where a low-level disinfectant will be suitable, resulting in ...
Manufactured by:Neupulse based inLenton, UNITED KINGDOM
Neupulse is a discreet, wrist-worn medical device designed to help reduce the frequency and intensity of motor and vocal tics in people with Tourette Syndrome. Backed by clinical research and developed with leading neuroscientists, Neupulse gives you a way to manage tics on your terms - anytime, ...
Manufactured by:Clear Surgical based inEdinburgh, UNITED KINGDOM
The days when medical and healthcare professionals had to make do with inadequate illumination are finally over. The totally reliable, highly innovative and easy to use Oplight enables the specialist to position a powerful light source anywhere they want, even in the darkest cavity. With the Oplight from Clear Surgical, you can have illumination how you want, where you want and for as long as you ...
Manufactured by:Acurable Limited based inLondon, UNITED KINGDOM
Clinically validated: AcuPebble SA100 automated sleep apnea test was validated in a powered clinical trial at the NHS Royal Free London hospital. Validated sleep study result equivalent to ambulatory gold-standard (multi-channel polygraphy followed by manual specialist interpretation). Validated result based on AHI and ODI for both 3% and 4% desaturation criteria with high accuracy (94% PPV, 98% ...
Manufactured by:Novara Technologies Ltd based inHartley Wintney, UNITED KINGDOM
Cleanliness and conformity are critical to medical cable assemblies and the considerations go much further such as ensuring that suitable materials are chosen which are acceptable for interaction with humans whilst also being able to withstand harsh sterilisation ...
Manufactured by:Chemence Inc based in, GEORGIA (US) (USA)
Chemence’s catheter and tube set bonding adhesives provide medical device manufacturers with reliable assembly solutions while reducing the total cost of ownership in the manufacturing process. Our KRYLEX® ranges of adhesives for medical device assembly have been formulated to meet the stringent assembly challenges ...
Manufactured by:Novara Technologies Ltd based inHartley Wintney, UNITED KINGDOM
Medical device companies are actively developing an increasing interest in electronics for disposable devices. Disposable devices are designed to be sterilised and used only one time and as a result unit cost can often be reduced by using a wider choice of engineering ...
Manufactured by:Chemence Inc based in, GEORGIA (US) (USA)
KRYLEX medical device assembly adhesives have been proven to help manufacturers of needle assemblies to improve manufacturing efficiencies and increase reliability and confidence in their finished devices. Whether assembling microneedles, pen needles, insulin delivery systems, prefilled syringes or any other type of assembly, Chemence’s ...
Manufactured by:ACE based inTelford, UNITED KINGDOM
The ACE process supplies thousands of eye ophthalmic surgery blades every month, using medical grade Stainless Steel. ...