- Home
- Equipment
- usa california
- allograft
Refine by
Allograft Equipment Supplied In Usa California
17 equipment items found
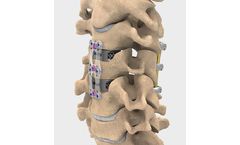
Manufactured by:Providence Medical Technology, Inc. based inPleasanton, CALIFORNIA (USA)
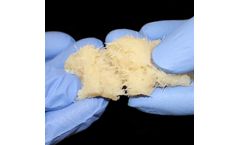
Machined from structural allograft bone, Graft-AB has a tapered tip designed to facilitate insertion into tight spaces, a parallel shape designed to provide uniform distraction, and superior and inferior teeth designed to prevent ...
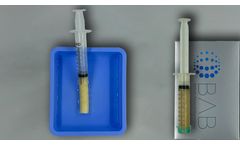
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
H-GENIN Crush-Mix is intended for use as a demineralized bone matrix for voids or gaps that are not intrinsic to the stability of the bony structure. Putty is indicated for the treatment of surgically created bone defects or bone defects created from traumatic injury. H-GENIN Crush-Mix provides a moldable consistency and can be mixed with blood or bone ...
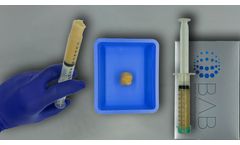
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
H-GENIN Putty is intended for use as a demineralized bone matrix for voids or gaps that are not intrinsic to the stability of the bony structure. Putty is indicated for the treatment of surgically created bone defects or bone defects created from traumatic injury. H-GENIN Putty provides a moldable consistency and can be mixed with blood or bone ...
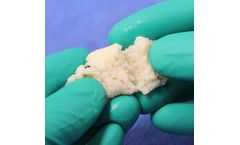
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
Demineralized Bone Matrix (DBM) is produced from ground cortical bone and contains osteoinductive proteins. It is intended to be used to fill bone defects and ...
by:Aurora Spine, Inc. based inToronto, ONTARIO (CANADA)
ZIP Graft is a cancellous allograft designed specifically for use with the ZIP® MIS Interlaminar Fusion Systems within the US. It is freeze-dried with a five-year shelf life. ZIP Graft is gamma-irradiated and packaged sterile for immediate use. All tissue and blood samples are tested for infectious diseases by CLIA Certified Labs. All of the testing and processing of donated ...
Manufactured by:NuVasive, Inc. based inSan Diego, CALIFORNIA (USA)
ACP is procedurally integrated with versatile instrumentation, a complete interbody portfolio featuring advanced porous materials, traditional PEEK and allograft, biologics, the Maxcess C retractor and enabling ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
It is comprised of cancellous bone particles with preserved cells combined with demineralized cortical particles and fibers all processed via Aziyo’s proprietary gentle VBM processes. OsteGro Allograft Bone Matrix, the second product in this family, is a sterile bone matrix comprised of cancellous particles and demineralized cortical ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
The natural allograft option that is as close to nature as possible. SimpliDerm® is a pre-hydrated human acellular dermal matrix with a sterility assurance level (SAL) of 10-6 and requires a minimal 2-minute sterile rinse for convenient intraoperative use.1 By maintaining a more intact matrix, SimpliDerm enhances and supports the angiogenic process, provides a more pliable ...
Manufactured by:Kinamed Incorporated based inCamarillo, CALIFORNIA (USA)
CarboJet CO2 Bone Preparation System is Quickly provides a cleaner and drier bone bed for a superior cement ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
ViBone is comprised of cancellous bone particles with preserved cells and demineralized cortical bone particles produced via Aziyo’s gentle proprietary VBM ...
Manufactured by:Spinal Elements, Inc. based inCarlsbad, CALIFORNIA (USA)
Viable Cell Population. An average of 1.5 million viable osteogenic cell population per cc of matrix are preserved via a non-DMSO cryoprotectant2. Endogenous bone cells remain attached via advanced processing techniques. Viable endogenous bone cells support osteogenic healing ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
Viable Bone Matrix for Spine and Orthopedic Procedures. Fiber Viable Bone Matrix: Fiber Viable Bone Matrix (VBM) is made of cancellous bone particles with preserved cells combined with demineralized cortical fiber produced via Aziyo’s gentle proprietary VBM ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Covered by Medicare, Prospera is a transplant rejection assessment test that uses a simple blood draw to evaluate the risk of rejection of a transplanted kidney. Through the use of advanced cell-free DNA technology, Prospera increases a provider’s ability to identify otherwise undetected rejection that might lead to kidney loss. Catching transplant rejection as soon as possible can help ...
Manufactured by:Cell Applications, Inc. based inSan Diego, CALIFORNIA (USA)
Human Aortic Endothelial Cells (HAOEC) provide an excellent model system to study all aspects of cardiovascular function and disease, and they have been utilized in dozens of research publications to study diabetes-associated complications related to cardiovascular function, investigate mechanisms of immune response and graft rejection, study endothelial dysfunction caused by air pollution, ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
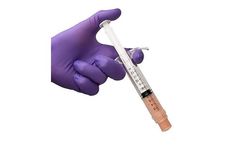
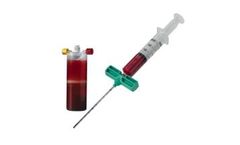
The system includes all the components to ASPIRATE blood and bone marrow, easily PROCESS the disposable system, and produce an autologous PRP output* to HYDRATE the surgeon’s choice of autograft and/or allograft ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
The system includes all the components to DRAW blood, ASPIRATE bone marrow, easily PROCESS the disposable system, and produce an autologous PRP output to HYDRATE the surgeon’s choice of autograft and/or allograft ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
Bonus Synthetic Bone Graft Substitute granules are resorbable, osteoconductive matrices consisting of a thin, 2–10 micron layer of hydroxyapatite over a calcium carbonate core. Bonus Synthetic Bone Graft Substitute has been indicated as a bone graft substitute that resorbs and is replaced with bone during the healing ...