- Home
- Equipment
- usa california
- biocompatibility
Refine by
Biocompatibility Equipment Supplied In Usa California
50 equipment items found
Manufactured by:Reva Medical LLC based inSan Diego, CALIFORNIA (USA)
Tyrocore™ is REVA’s proprietary polymer invented specifically for bioresorbable scaffolds. ...
Manufactured by:Prellis Biologics, Inc. based inSan Francisco, CALIFORNIA (USA)
Prellis’ proprietary holographic laser printing technology enables rapid construction of high-resolution biocompatible scaffolds. Prellis bioprints complex micron-feature sized scaffolds up to 400 times faster than other laser printing technologies. Prellis holographic printing is fully integrated with internally developed software, biomaterial chemistry, and optical ...
Manufactured by:Boston Scientific based inMarlborough, MASSACHUSETTS (USA)
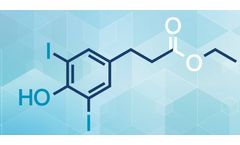
LC Bead is a controlled embolization system intended for the purpose of embolizing the blood vessels of a variety of hypervascularized tumors and arteriovenous malformation. LC Bead is a preformed, deformable microsphere consisting of a biocompatible, sulphonate- modified, N-Fil hydrogel. LC Bead is cleared by FDA for the embolization of hypervascularised tumors and arteriovenous ...
Manufactured by:MRIaudio based inCarlsbad, CALIFORNIA (USA)
These lightweight earpieces deliver 29 decibels of noise attenuation while still allowing patients to enjoy their favorite music or podcast. To ensure safety we offer disposable tips that are latex free and biocompatibility tested. ...
Manufactured by:STAAR Surgical Company based inLake Forest, CALIFORNIA (USA)
STAAR Surgical's phakic IOL for myopia, hyperopia and astigmatism. The Visian ICL is made from Collamer, a biocompatible material created and used exclusively by STAAR Surgical. This lens is able to treat a large range of refractive error varying by ...
Manufactured by:STC Material Solutions based inSanta Ana, CALIFORNIA (USA)
STC Material Solutions played a key role in developing customized feedthroughs for medical implants. All medical feedthroughs are designed and manufactured using only biocompatible materials. Our engineering team has years of expertise in material selection. This ensures our feedthroughs are not only biocompatibile, but also optimized for production to control costs. STC Material ...
Manufactured by:Shape Memory Medical Inc. based inSanta Clara, CALIFORNIA (USA)
The porous embolic scaffold supports the rapid formation of small interconnected clots throughout its structure. The biocompatible and non-inflammatory polymer promotes rapid conversion to organized thrombus, followed by collagen deposition without chronic active inflammation, which leads to a stable occlusion. ...
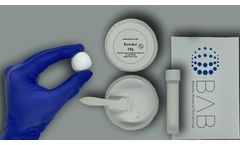
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
It functions as an osteogenic stimulus to which the patient’s bone marrow can be added, prior to implantation. Bi-Ostetic Foam™ is safe and has excellent biocompatibility. After it is implanted, it resorbs and is later replaced by natural bone. Bi-Ostetic Foam™ is a natural choice for sparing patients the trauma of autograft ...
Manufactured by:Starch Medical, Inc. based inSan Jose, CALIFORNIA (USA)
SuperClot® Absorbable Polysaccharide Hemostat is a medical device composed of absorbable modified polymer (AMP®) particles and delivery applicator. AMP® particles are biocompatible, non-pyrogenic and derived from purified plant starch. The device contains no human or animal components. SuperClot® is intended as an absorbable hemostat system to control bleeding ...
Manufactured by:Bioplate based inPlacentia, CALIFORNIA (USA)
With a focus on facilitating natural tissue regeneration, Bioplate's sterile kits provide a cost-effective solution that does not compromise on the quality. Biocompatible materials such as titanium are utilized in these kits to ensure safety and effectiveness, although surgeons are advised to evaluate the types of fixation devices based on the specific case and patient ...
Manufactured by:Shape Memory Medical Inc. based inSanta Clara, CALIFORNIA (USA)
The porous embolic scaffold supports the rapid formation of small interconnected clots throughout its structure. The biocompatible and non-inflammatory polymer promotes rapid conversion to organized thrombus, followed by collagen deposition without chronic active inflammation, which leads to a stable occlusion.‡ The anchor coil offers stability in higher-flow locations and ...
by:Molecular Matrix, Inc. based inWest Sacramento, CALIFORNIA (USA)
Osteo-P™ Bone Graft Substitute (BGS) is a non-mineralized, synthetic bone void filler consisting of a hyper-crosslinked carbohydrate polymer. It is highly porous, biocompatible, biodegradable, and osteoconductive. Osteo-P™ BGS is intended for the filling of bone defects created surgically or as a result of traumatic injury. When placed in direct contact with patient ...
Manufactured by:Shape Memory Medical Inc. based inSanta Clara, CALIFORNIA (USA)
The porous embolic scaffold of the shape memory polymer supports the rapid formation of small interconnected clots throughout its structure. The biocompatible and non-inflammatory polymer promotes the rapid conversion to organized thrombus, followed by collagen deposition without chronic active inflammation, which leads to a stable occlusion. ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
It functions as an osteogenic stimulus to which the patient’s bone marrow can be added to, prior to implantation. Bi-Ostetic Bioactive Glass Foam™ is safe and has excellent biocompatibility. After it is implanted, it resorbs and is later replaced by natural bone. Bi-Ostetic Bioactive Glass Foam™ is a natural choice for sparing patients the trauma of autograft ...
Manufactured by:EnColl Corporation based inFremont, CALIFORNIA (USA)
PPAB is a collagen fibril based formulation containing tetracycline hydrochloride (2 mg of Tetracycline) in 25 mg of collagen fibrils. It has a dual mode of action, via the active agent, Tetracycline, and the vehicle, high purity, biocompatible Type-I collagen, which are used. This very unique feature of PPAB enables the active agent as well as the vehicle to be able to work ...
Manufactured by:Boston Scientific based inMarlborough, MASSACHUSETTS (USA)
Bead Block is an embolic device intended for the purpose of embolising the blood vessels of a variety of hypervascularised tumours (e.g. uterine fibroids) and arteriovenous malformations. Bead Block is a preformed, deformable microsphere consisting of a biocompatible, acrylamido polyvinyl alcohol macromer. Bead Block compressible microspheres are used in embolisation therapy. ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
Cem-Ostetic® is a neutral pH bone putty that contains biocompatible calcium salts that have been used for decades in orthopedic surgery. These materials are often used to provide an additional source of bone to help the patient heal faster. Berkeley Advanced Biomaterials formulates the chemistry and microstructure to enhance bone regeneration, provide optimal ...
Manufactured by:Starch Medical, Inc. based inSan Jose, CALIFORNIA (USA)
SealFoam® Absorbable Polysaccharide Hemostat is a medical device composed of AMP® (absorbable modified polymer) particles which are very hydrophilic, biocompatible, non-pyrogenic and derived from purified plant starch.The device contains no human or animal components. SealFoam® is a naturally adhesive absorbable hemostatic pad designed to control bleeding during ...
Manufactured by:Bioplate based inPlacentia, CALIFORNIA (USA)
The Osteopore Bioresorbable Bone Scaffold is specifically designed to address craniotomy gaps, promoting improved aesthetics, reduced pain, and enhanced patient satisfaction. Made from polycaprolactone, this biocompatible implant fully resorbs over a span of 18-24 months via in vivo hydrolysis. Its 3D-printed structure mimics cancellous bone, encouraging vascularization and ...
Manufactured by:EnColl Corporation based inFremont, CALIFORNIA (USA)
Over 100 years of research, experimentation and use has demonstrated that calcium sulphate enhances bone regeneration and is totally biocompatible and and resorbable. At the same token, high purity type-I collagen DMBM (xenograft) also is essential for tissue regeneration and remodeling. Osseomold consists of both materials admixed in proper proportions to give the best ...