- Home
- Equipment
- usa california
- bone marrow
Show results for
Refine by
Bone Marrow Equipment Supplied In Usa California
25 equipment items found
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
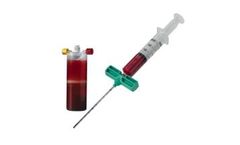
Designed to process a mixture of autologous whole blood and bone marrow aspirate, the BioCUE BBMA Concentration System represents an evolution in this technique. The system includes all the components to ASPIRATE blood and bone marrow, easily PROCESS the disposable system, and produce an autologous PRP output* to HYDRATE the ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
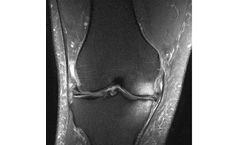
Bone Marrow Lesions (BML) are MRI-visible defects in the subchondral bone. BML can only be seen on fat-suppressed MRI sequences (T2FS, PDFS, etc.) where they appear as a hazy white area against the background of darker bone. Pathologists have shown that BML represent a healing response to trauma such as micro trabecular fractures ...
Manufactured by:Senti Biosciences based inSouth San Francisco, CALIFORNIA (USA)
Pursuing an Unmet Need in the Treatment of AML - SENTI-202 is designed to target and eliminate acute myeloid leukemia cells while sparing healthy bone marrow. Our vision is a potential medicine that does not rely on an invasive bone marrow ...
Manufactured by:Biocardia, Inc. based inSunnyvale, CALIFORNIA (USA)
Where the CardiAMP Cell Therapy System uses a patient’s own cells, the CardiALLO system uses culture-expanded, bone marrow-derived mesenchymal stem cells from a universal donor. This allows the cells to be obtained "off the shelf" from a pharmacy, negating the need for a bone marrow aspirate for the patient and providing an ...
Manufactured by:AllCells based inAlameda, CALIFORNIA (USA)
Hematopoietic stem and progenitor cells (HSPCs) are the key stem cell population in the bone marrow capable of self-renewal and multilineage differentiation into mature blood cell types. CD34+ HSPCs can be isolated from mobilized peripheral blood, bone marrow, and umbilical cord blood. Clinical HSPC transplantation is used to ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
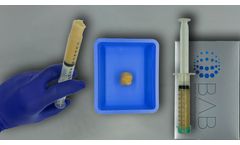
H-GENIN Putty is intended for use as a demineralized bone matrix for voids or gaps that are not intrinsic to the stability of the bony structure. Putty is indicated for the treatment of surgically created bone defects or bone defects created from traumatic injury. H-GENIN Putty provides a moldable consistency and can be mixed with blood or ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
H-GENIN Crush-Mix is intended for use as a demineralized bone matrix for voids or gaps that are not intrinsic to the stability of the bony structure. Putty is indicated for the treatment of surgically created bone defects or bone defects created from traumatic injury. H-GENIN Crush-Mix provides a moldable consistency and can be mixed with blood ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
Bi-Ostetic Foam™ is a sterile bone graft composed of highly purified fibrillar Type I bovine collagen and Bi-Ostetic™ resorbable 60% hydroxyapatite and 40% tricalcium phosphate granules. It functions as an osteogenic stimulus to which the patient’s bone marrow can be added, prior to implantation. Bi-Ostetic Foam™ is safe ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
Bi-Ostetic Bioactive Glass Foam™ is a sterile bone graft composed of highly purified fibrillar Type I bovine collagen, 45S5 bioactive glass granules and Bi-Ostetic™ resorbable 60% hydroxyapatite and 40% tricalcium phosphate granules. It functions as an osteogenic stimulus to which the patient’s bone marrow can be added to, ...
Manufactured by:Stemedica Cell Technologies, Inc. based inSan Diego, CALIFORNIA (USA)
itMSCs (ischemia-tolerant mesenchymal stem cells) are “trophic” cells that circulate throughout the body and release proteins and growth factors into the damaged tissue, rescuing damaged cells from death and creating the right conditions for the newly mobilized cells to proliferate and repair. Stemedica’s itMSCs are extracted from donated bone ...
Manufactured by:AllCells based inAlameda, CALIFORNIA (USA)
AllCells’ GMP Mobilized leukopaks are apheresis products collected from healthy donors treated with FDA-approved drugs to stimulate mobilization of CD34+ hematopoietic stem and progenitor cells from the bone marrow to the peripheral blood. This procedure results in large-scale yields of CD34+ cells. Peripheral blood is collected from IRB-consented donors ...
Manufactured by:AllCells based inAlameda, CALIFORNIA (USA)
Mobilized Leukopaks are apheresis products collected from healthy donors treated with FDA-approved drugs to stimulate the migration of CD34+ hematopoietic stem and progenitor cells (HSPCs) from the bone marrow niche into the circulation. Mobilized peripheral blood, enriched in single-donor CD34+ HSPCs is collected through leukapheresis using the Spectra ...
Manufactured by:Biocardia, Inc. based inSunnyvale, CALIFORNIA (USA)
The investigational CardiAMP™ Cell Therapy is designed to be a comprehensive biotherapeutic heart failure solution, incorporating: a proprietary molecular diagnostic to characterize the potency of a patient’s own bone marrow cells and determine if they are an optimal candidate for therapy. a point of care processing platform to prepare cells at the ...
Manufactured by:Fate Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
Multiple myeloma is a hematologic malignancy characterized by the proliferation of malignant plasma cells. In multiple myeloma, malignant plasma cells accumulate in the bone marrow and produce abnormal antibodies called M proteins, which can cause kidney damage, bone destruction, and impaired immune function. While multiple approved drugs with ...
Manufactured by:PAVmed Inc. based inNew York, NEW YORK (USA)
The intraosseous route provides a means for infusing fluids, medications, and other substances directly into the bone marrow cavity which communicates with the central venous circulation via nutrient and emissary veins. This route, which has been used for decades, is well established and has been shown to bioequivalent to the intravenous route. PortIO is a novel, ...
Manufactured by:Access Biologicals LLC based inVista, CALIFORNIA (USA)
Human AB serum is regularly used for tissue engineering, transplantation and expansion of T-cells, cell therapy applications and cell line testing for PBMCs, bone marrow, CD9, CD3 and CD33. In addition, Human Male AB Serum is the main growth component used is CAR-T cell therapy applications. Our Human Male AB serum is collected via a multi-step process that is ...
Manufactured by:EntroGen, Inc. based inWoodland Hills, CALIFORNIA (USA)
Many hematologic malignancies carry characteristic chromosomal translocations believed to play an important role in the pathogenesis of these tumors. Chromosomal translocations found in leukemias and lymphomas either fuse enhancer/promoter regions of one gene to another, causing aberrant expression, or by fusing a part of one gene to that of another, resulting in disruption of the normal function ...
by:Cytori Therapeutics Inc. based inSan Diego, CALIFORNIA (USA)
Cytori is developing cell therapies that harness the unique attributes of living cells that are present in an adult human patient’s own adipose (fat) tissue, also known as Adipose-Derived Regenerative Cells ...
Manufactured by:EntroGen, Inc. based inWoodland Hills, CALIFORNIA (USA)
EntroGen NGS Targeted Hotspot Panel is a comprehensive pan-cancer assay designed to detect clinically relevant hotspot mutations in solid tumors. Utilizing a robust next-generation sequencing (NGS) platform, labs are able to simultaneously analyze several different tumor types by batching up to 12 samples on a single run*. NGS Targeted Hotspot Panel (THSP) is compatible with fresh frozen, and ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
Agilon Strip Collagen Enhanced Bone Grafting Product is the latest addition to the Biogennix bone grafting product family. Developed with Biogennix proprietary TrelCor technology, Agilon Strip consists of resorbable, osteoconductive TrelCor granules densely packed in a matrix of type 1 bovine ...