- Home
- Equipment
- usa california
- cardiovascular
Refine by
Cardiovascular Equipment Supplied In Usa California
78 equipment items found
Manufactured by:Jant Pharmacal Corporation based inEncino, CALIFORNIA (USA)
Debating on whether the cost is worth the long term investment for your lab? Jant Pharmacal offers a complimentary ROI Assessment to help you determine the right solution for your lab’s needs. We offer ROI proposals at no cost to help lab managers determine the feasibility of pursuing new technology and service offerings for their lab. ...
Manufactured by:Vetclassics based inTemecula, CALIFORNIA (USA)
For use in dogs over the age of 12 weeks. Supports normal function of the heart and cardiovascular ...
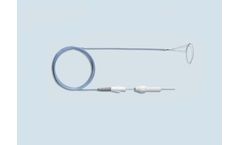
Manufactured by:PFM Medical, Inc. based inCarlsbad, CALIFORNIA (USA)
The Nit-Occlud PDA occlusion system is the ideal choice for small to medium sized patent ductus arteriosus types. The tightly compact nitinol windings provide safe and effective closure for its patients. This highly flexible and adaptive coil system comes pre-loaded in the delivery system is easily repositionable and retrievable to benefit ...
Manufactured by:Ralco s.r.l based inBiassono (MB), ITALY
Fixed C-Arm/ Cardiovascular, Mobile C-Arm/ Mobile Surgery Automatic Collimators. Single layer, round and elliptic field, automatic collimation system intended for installation in connection with an Image Intensifier mounted on mobile or stationary C-arm equipment. This device has been designed and manufactured for skeletal and cardiovascular ...
Manufactured by:Ralco s.r.l based inBiassono (MB), ITALY
Fixed C-Arm/ Cardiovascular, Mobile C-Arm/ Mobile Surgery Automatic Collimators. Single layer, square field, automatic collimation system intended for installation on stationary or mobile C-arm equipment in connection with a digital image receptor. This device has been designed and manufactured for skeletal and cardiovascular ...
Manufactured by:Khloris Biosciences, Inc. based inMountain View, CALIFORNIA (USA)
Khloris' iPSCs are optimized to generate high-functioning cardiomyocytes, enabling development of an allogeneic source of donor cells for patients with damaged heart ...
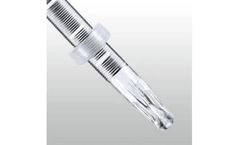
Manufactured by:PFM Medical, Inc. based inCarlsbad, CALIFORNIA (USA)
The Multi-Snare Device is a patented, highly efficient multiple loop retrieval snare with a dual-plane design to grasp objects from numerous angles. Designed with various reliable pull-force strengths, the kink resistant nitinol dual loop system grants the ability to grasp, manipulate or assist from any angle or vessel sidewall. The Multi-Snare MAX Device has been designed with the same ...
Manufactured by:BiosPacific, Inc. based inEmeryville, CALIFORNIA (USA)
Catalog number: A66014, product description: ACTH Monoclonal Antibody, Purified, lot number: [lot specific], immunogen: Human ACTH (18-39), source: Murine, form: Liquid, purification method: Protein A affinity chromatography, buffer: 0.1 M BBS, pH 8, isotype: IgG1, Kappa, concentration: [lot specific] mg/mL, specificity: Recognizes Human ACTH (18-39) and Human ACTH (1-39). No, cross reactivity to ...
Manufactured by:BiosPacific, Inc. based inEmeryville, CALIFORNIA (USA)
Product: ACTH N-Terminal Monoclonal Antibody. Source: Cell culture. Immunogen: KLH conjugate. Purification Method: Protein A affinity chromatography. Buffer: PBS, pH 7.2. Preservative: 10mM NaN3. Subclass: IgG1. Storage Conditions: 2-8o C short term, -20o C long term. Specificity: Synacthen (1-24 ACTH) and has no cross reactivity with CLIP (ACTH 17-39). This antibody has an affinity constant ...
Manufactured by:Gilead based inFoster City, CALIFORNIA (USA)
Ambrisentan 5 mg and 10 mg. Letairis (ambrisentan) tablets, for oral use. ...
Manufactured by:Diazyme Laboratories, Inc. based inPoway, CALIFORNIA (USA)
Diazyme's HDL-Cholesterol Assay is a direct dual liquid stable reagent system. Convenient instrument specific packaging options including Roche™ Hitachi 917 series, Beckman AU (400/600/640/680), Beckman Synchron CX, LX and DXC. The assay utilizes Diazyme's proprietary PVSPEGME coupled technology which provides outstanding performance. The assay features a linear range of 1.06 to 184.8 ...
Manufactured by:Diazyme Laboratories, Inc. based inPoway, CALIFORNIA (USA)
Diazyme's LDL-Cholesterol Assay is a direct dual liquid stable reagent system. Reagent transfer is eliminated for most chemistry systems with a choice of convenient instrument specific packaging options including Roche™ Hitachi 917 series, Beckman AU (400/600/640/680), Beckman Synchron CX, LX and DXC. The assay utilizes Diazyme's proprietary PVSPEGME coupled technology which provides ...
Manufactured by:Vetclassics based inTemecula, CALIFORNIA (USA)
For dogs who are active, pregnant, whelping, nursing, or recovering from surgery, trauma, anemia, or parasitic events. Helps support the formation of hemoglobin and myoglobin. Also helps support energy and ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
Designed to be gentle on the aorta and easy to insert, Soft-Flow®* arterial cannulae help you proceed with confidence during cardiac surgery procedures. As the first diffusion tip arterial cannulae on the market, the Soft-Flow was the cannula of choice for many cardiac surgeons. Working with MC3 Cardiopulmonary, we’ve made it possible to bring Soft-Flow arterial cannulae back to the ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
Simplify your circuit with the first oxygenator featuring integrated monitoring. The Nautilus™* Smart ECMO Module improves long-term gas transfer1 while providing real-time device performance data accessible from an intuitive touch screen. ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The Crescent™* catheter is the first FDA-cleared, jugular dual lumen long-term ECMO catheter. It allows for more accurate placement with just one cannulation site, delivers enhanced flow dynamics, and helps maintain optimal flow† once placed. ...
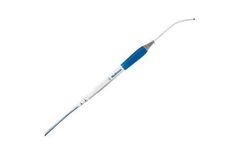
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The Cardioblate Surgical Ablation Pen is intended to ablate cardiac tissue during cardiac surgery using radiofrequency ...
Manufactured by:Abbott Laboratories based inAbbott Park, ILLINOIS (USA)
Backed by a 15-year track record of performance in the U.S.,1 the Amplatzer Duct Occluder can accommodate large PDAs with a single device—which can aid in minimizing the complexity of the ...
Manufactured by:Abbott Laboratories based inAbbott Park, ILLINOIS (USA)
The Advisor HD Grid Mapping Catheter, Sensor Enabled™, is a steerable and insulated electrophysiology mapping catheter with a grid-patterned electrode configuration. The 16 electrodes can be placed where you need, offering high-density wave bipole recordings along and across the spines, direction independent mapping1 and faster data collection in a given ...
Manufactured by:Abbott Laboratories based inAbbott Park, ILLINOIS (USA)
Used in more than 1,000,000 procedures, the Agilis™ NxT steerable introducer provides outstanding agility and stability during catheter access and ...