- Home
- Equipment
- usa california
- clinical outcome assessment
Refine by
Clinical Outcome Assessment Equipment Supplied In Usa California
7 equipment items found
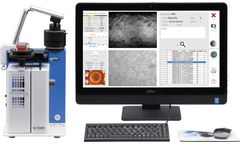
Manufactured by:XILIS, Inc. based inDurham, NORTH CAROLINA (USA)
Xilis’ MicroOrganoSphereTM powers the only assay which can provide oncologists with accurate results of drug sensitivity on a patient’s own tumor within the clinical decision-making window - finally realizing the promise of precision medicine. Xilis is developing a panel of disease specific assays systematically testing the most important standard-of-care drugs within equipoise or ...
Manufactured by:Konan Medical USA, Inc. based inIrvine, CALIFORNIA (USA)
Designed to improve patient outcomes, CellChek D+ redefined specular microscopy in Eyebanking. It was quickly and widely adopted by many of the world’s finest Eye ...
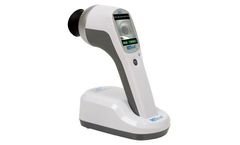
Manufactured by:Konan Medical USA, Inc. based inIrvine, CALIFORNIA (USA)
RETeval is the only FDA 510(k) cleared, non-mydriatic* full-field ERG device that is easier to use than you might think. Many diseases and disorders affect various cell populations in the retina, and ERG testing provides an objective, non-invasive method of evaluating retinal function. Hand-held, battery-powered & easy to use anywhere, all day, with easy interpretation aided by a 500+ ...
Manufactured by:Active Life Scientific, Inc. based inSanta Barbara, CALIFORNIA (USA)
OsteoProbe is a portable handheld impact microindenter that measures the ability of bone material to resist indentation. Unlike radiological or imaging methods (X-ray, DXA and CT) that measure bone mineral density and bone mineral structure, OsteoProbe measures bone material quality, which is an independent component of bone health. In the United States, OsteoProbe is an Investigational Device. ...
Manufactured by:DxTerity Diagnostics Inc. based inRancho Dominguez, CALIFORNIA (USA)
DxTerity’s IFN-1 Test uses a simple fingerstick blood collection conducted at your doctor's office. Measure your IFN-1 levels and know your risks for SLE disease progression. Take back control of your ...
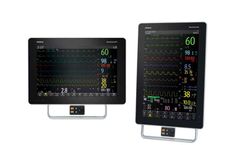
Manufactured by:Mindray North America based inMahwah, NEW JERSEY (USA)
The BeneVision N19 and N22 are part of Mindray’s premier patient monitoring solution designed to enhance patient care and support timely and accurate clinical assessment across Emergency, Critical Care, and Operating Room environments. With an expansive set of multi-parameter measurements and specialized Clinical Assistance Applications (CAAs), the N19 and N22 provide a fully modular and ...
Manufactured by:Jant Pharmacal Corporation based inEncino, CALIFORNIA (USA)
The RAMP® NT-proBNP test is an accurate, quantitative and rapid diagnostic test that aids in the diagnosis and assessment of severity in individuals suspected of having congestive heart failure (CHF) and can aid in the risk stratification of patients with heart failure. The RAMP® NT-proBNP test is ideally suited for point of care testing and laboratory use by healthcare professionals. ...