- Home
- Equipment
- usa california
- coronavirus
Show results for
Refine by
Coronavirus Equipment Supplied In Usa California
21 equipment items found
Manufactured by:Liferiver Bio-Tech (United States) Corp. based inLa Jolla, CALIFORNIA (USA)
LightCycler 1.0 (Internal control can't be used for this system); LightCycler 2.0; PE5700, MJ-Opticon & other single color systems; ABI7000, ABI7300, ABI7500, ABI7900, ABI StepOne, StepOne plus, MJ-Opticon2, MJ-chromo4, MX3000P, MX3005P, Smart Cycler II, Rotor-Gene 6000, LightCycler 480, CFX 96, Slan 96, MIC, iCycler iQ4, iCycler iQ5 & other multi-color ...
Manufactured by:Liferiver Bio-Tech (United States) Corp. based inLa Jolla, CALIFORNIA (USA)
LightCycler 1.0 (Internal control can't be used for this system); LightCycler 2.0; PE5700, MJ-Opticon & other single color systems; ABI7000, ABI7300, ABI7500, ABI7900, ABI StepOne, StepOne plus, MJ-Opticon2, MJ-chromo4, MX3000P, MX3005P, Smart Cycler II, Rotor-Gene 6000, LightCycler 480, CFX 96, Slan 96, MIC, iCycler iQ4, iCycler iQ5 & other multi-color ...
Manufactured by:Liferiver Bio-Tech (United States) Corp. based inLa Jolla, CALIFORNIA (USA)
LightCycler 1.0 (Internal control can't be used for this system); LightCycler 2.0; PE5700, MJ-Opticon & other single color systems; ABI7000, ABI7300, ABI7500, ABI7900, ABI StepOne, StepOne plus, MJ-Opticon2, MJ-chromo4, MX3000P, MX3005P, Smart Cycler II, Rotor-Gene 6000, LightCycler 480, CFX 96, Slan 96, MIC, iCycler iQ4, iCycler iQ5 & other multi-color ...
Manufactured by:Pfizer Inc. based in, NEW YORK (USA)
Food and Drug Administration (FDA), but has been authorized for emergency use by FDA under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19) for use in individuals 16 years of age and older. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency ...
Manufactured by:Gilead based inFoster City, CALIFORNIA (USA)
100 mg for injection VEKLURY is a prescription medicine used to treat adults and children 12 years of age and older and weighing at least 88 pounds (40 kg) with coronavirus disease 2019 (COVID-19) requiring ...
Manufactured by:Coretests, Inc. based inCarlsbad, CALIFORNIA (USA)
COVID-19 Neutralizing Ab Test is used for qualitative detection of the Neutralizing antibodies in patients who have recovered from Novel Coronavirus Pneumonia or those who have been vaccinated. COVID-19 Neutralizing Ab Test: sensitivity -99.2%,specificity - 99.3%,total agreement - 99.3%. Fast to read result, High accuracy, Convenient to use ...
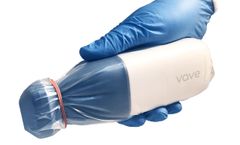
Manufactured by:Vave Health, Inc. based inSanta Clara, CALIFORNIA (USA)
Vave supports coronavirus diagnosis and monitoring. Incredibly portable - no moving patients or bulky equipment. Save your PPE - with VaveCast, you can cast your scanning anywhere in real-time, requiring only one person in the room. Wireless and Waterproof — it’s never been easier to sterilize your ultrasound equipment between ...
Manufactured by:Lumiquick Diagnostics, Inc based inSanta Clara, CALIFORNIA (USA)
QuickProfile 2019-nCoV IgG/IgM Combo Test Card is an immunochromatography based one step in vitro test. It is designed for the rapid qualitative determination of IgG and IgM antibodies to 2019 novel coronavirus (2019-nCoV, SARS-CoV-2) in human serum, plasma, or whole blood. Rapid 2019-nCoV IgG/IgM Combo Test Card is a supplemental rapid screening tool for symptomatic or ...
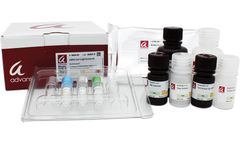
Manufactured by:Advansta Inc. based inSan Jose, CALIFORNIA (USA)
In December of 2019, the SARS-CoV-2 virus, which causes the disease COVID-19, was first detected in Wuhan, China. The SARS-CoV-2 virus is a single-stranded RNA coronavirus that causes respiratory infections. The rapid spread of COVID-19 has resulted in a global pandemic which has caused an unprecedented social and economic disruption to daily ...
Manufactured by:Anchor Molecular based inPleasanton, CALIFORNIA (USA)
The virus carries known target sequences (Wuhan-Hu-1, NC_045512.2) used in more than 160 molecular tests worldwide, including the coding regions for ORF1ab, RDRP, S, E, N, M, ORF8 and unique regions among the coronavirus family. For the control of nucleic acids derived from human cells, this virus also carries a 65-base human RNase P sequence used by the Centers for Disease ...
Manufactured by:Gold Standard Diagnostics based inDavis, CALIFORNIA (USA)
The GSD NovaType Detect + Select K417N SARS-CoV-2 is a research use only (RUO) real-time reverse transcription PCR (RT-PCR) assay designed for the simultaneous qualitative detection of SARS-CoV-2 (Severe acute respiratory syndrome coronavirus 2) genomic RNA and identification of the spike (S) gene mutation K417N. It is intended to aid in the identification of SARS-CoV-2 variant ...
Manufactured by:Meissa Vaccines, Inc. based inRedwood City, CALIFORNIA (USA)
Meissa is developing a live attenuated COVID-19 vaccine candidate, MV-014-212, to induce mucosal and systemic immunity and protect against SARS-CoV-2, the novel coronavirus that causes COVID-19. Like Meissa’s RSV vaccine candidate, MV-014-212 offers significant potential advantages for global deployment, including needle-free intranasal administration, a single ...
Manufactured by:AIVITA Biomedical, Inc. based in, CALIFORNIA (USA)
AIVITA Biomedical is using its patient-specific vaccine platform to develop a rapidly scalable vaccine candidate, AV-COVID-19, for the novel coronavirus (SARS-CoV-2). AIVITA’s personalized vaccine begins with a basic blood draw, from which the recipient’s own immune cells are extracted and loaded with SARS-CoV-2 antigen. The resulting personalized treatment is ...
Manufactured by:IGeneX based inMilpitas, CALIFORNIA (USA)
IGeneX is now offering the COV5T SARS-CoV-2 Vaccine Response Test Panel, which tests for antibody response and T-cell response. This comprehensive test panel gives patients the ability to monitor their overall response to the ...
Manufactured by:GeneTex, Inc. based inIrvine, CALIFORNIA (USA)
Optimal dilutions/concentrations should be determined by the ...
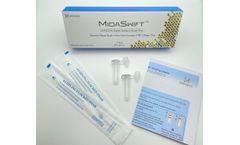
Manufactured by:Nirmidas Biotech, Inc. based inPalo Alto, CALIFORNIA (USA)
Nirmidas Biotech Inc. has developed an antigen test aiming for FDA EUA, MidaSwift™ SARS-CoV-2 Antigen Detection Kit, for the qualitative detection of the Nucleocapsid antigen from SARS-CoV-2. The test is curently for Research Use Only (RUO), for use with anterior nares (lower nostril) swab specimens. It can potentially be used at the Point-of-Care (POC) by licensed profesionals at CLIA ...
Manufactured by:Able Diagnostics, Inc. based inSan Diego, CALIFORNIA (USA)
The 1st Point-of-Care (POC) Antibody Test for COVID-19 with Fingerstick Whole Blood Authorized by FDA!. Click the link to review FDA NEWS RELEASE on Sept 23, 2020 in details: "FDA Authorizes First Point-of-Care Antibody Test for COVID-19" about Assure COVID-19 IgG/IgM Rapid Test Device. This authorization means that Fingerstick Blood Samples can now be tested in POC settings like Doctor's ...
Manufactured by:Metrex Research, LLC. based inOrange, CALIFORNIA (USA)
Durable, non-woven towelettes that offer quick, easy-to-use, time-saving convenience killing organisms in only 3 minutes. For use on hard non-porous surfaces of non-invasive medical ...
Manufactured by:Nirmidas Biotech, Inc. based inPalo Alto, CALIFORNIA (USA)
The test can be used for a variety of samples including serum, plasma, whole blood, dry blood spot, and saliva in a lab facility. It can be used to assess IgG and IgM levels in a sample with 100% sensitivity and 99.87% specificity without interferences from other diseases. Avidity pGOLD™ assay can tell if a COVID-19 infection is recent or remote greater than 6 months ...
Manufactured by:Metrex Research, LLC. based inOrange, CALIFORNIA (USA)
Convenient, ready-to-use surface disinfectant that is effective many harmful organisms. When used as directed, it will also effectively clean and disinfect non-critical and non-semicritical ...











![GeneTex - Model SARS-CoV-2 (COVID-19)-[HL5511] - Nucleocapsid Antibody GeneTex - Model SARS-CoV-2 (COVID-19)-[HL5511] - Nucleocapsid Antibody](https://d32zuqhgcrpxli.cloudfront.net/eyJidWNrZXQiOiJlZS1maWxlcyIsImtleSI6ImZpbGVzLzExMzU1Ny9wcm9kdWN0cy83Mjk0NTUvMTEzNTU3XzJfMjAyMDEwMjkwNTU3MDM0NTY5MTMzX3Jhdy5KUEciLCJlZGl0cyI6eyJyZXNpemUiOnsiZml0IjoiY29udGFpbiIsImJhY2tncm91bmQiOnsiciI6MjU1LCJnIjoyNTUsImIiOjI1NSwiYWxwaGEiOjF9LCJ3aWR0aCI6MjQwLCJoZWlnaHQiOjE0NX19fQ==)