- Home
- Equipment
- usa california
- covid rapid
Refine by
Covid Rapid Equipment Supplied In Usa California
6 equipment items found
Manufactured by:CTK Biotech, Inc. based inPoway, CALIFORNIA (USA)
The Positivia COVID-19 Ag Rapid Test External Control Kit is intended for use with the OnSite COVID-19 Ag Rapid Test (REF ...
Manufactured by:ACON Laboratories, Inc. based inSan Diego, CALIFORNIA (USA)
Detects IgG/IgM antibodies to SARS-CoV-2. Product Features: High Sensitivity and Specificity - Relative Sensitivity: 99.1% (95.12 – 99.98 %)*, Relative Specificity: 98.2% (96.29 – 99.27%)*, Accuracy: 98.5% (96.9 – 99.5%)*, *95% Confidence Intervals. Device Highlights: Test Cassette, Detects IgG and IgM, Small sample volume, Multiple sample formats, Fast results. Specimen ...
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
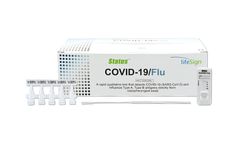
A rapid immunoassay for the simultaneous direct detection and differential diagnosis of SARS-CoV-2, Influenza Type A and Type B antigen from anterior nasal (AN) or nasopharyngeal (NP) swab specimens. Kit includes extraction buffer vials and flocked nasopharyngeal (NP) swabs for superior specimen collection and patient comfort, and provides a visual read-out in 15 minutes with no equipment needed! ...
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
The RightSign COVID-19 IgG/IgM Rapid Test Cassette is a lateral flow immunochromatographic assay for the detection of SARS-CoV-2 antibodies in venous whole blood, serum, or plasma. The COVID-19 IgG/IgM Rapid Test is intended for use as an aid in identifying individuals with an adaptive immune response (due to infection or ...
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
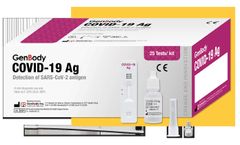
An immunochromatographic rapid diagnostic test (RDT) intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nasopharyngeal (NP) or anteriornasal (AN) swab specimens. Authorized for use under an Emergency Use Authorization (EUA) and for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate ...
Manufactured by:Able Diagnostics, Inc. based inSan Diego, CALIFORNIA (USA)
The 1st Point-of-Care (POC) Antibody Test for COVID-19 with Fingerstick Whole Blood Authorized by FDA!. Click the link to review FDA NEWS RELEASE on Sept 23, 2020 in details: "FDA Authorizes First Point-of-Care Antibody Test for COVID-19" about Assure COVID-19 IgG/IgM Rapid Test Device. This authorization means that Fingerstick ...