- Home
- Equipment
- usa california
- covid testing
Show results for
Refine by
Covid Testing Equipment Supplied In Usa California
19 equipment items found
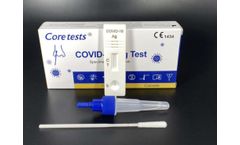
Manufactured by:Coretests, Inc. based inCarlsbad, CALIFORNIA (USA)
COVID-19 Neutralizing Ab Test is used for qualitative detection of the Neutralizing antibodies in patients who have recovered from Novel Coronavirus Pneumonia or those who have been vaccinated. COVID-19 Neutralizing Ab Test: sensitivity -99.2%,specificity - 99.3%,total agreement - 99.3%. ...
Manufactured by:CTK Biotech, Inc. based inPoway, CALIFORNIA (USA)
The Positivia COVID-19 Ag Rapid Test External Control Kit is intended for use with the OnSite COVID-19 Ag Rapid Test (REF ...
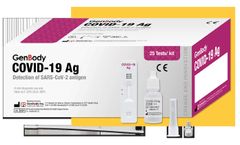
Manufactured by:Coretests, Inc. based inCarlsbad, CALIFORNIA (USA)
COVID-19 Ag Test is the chromatographic assay test used for qualitative detection of the COVID-19 nucleoprotein antigen in nasal swab specimen from, suspected patients may infected COVID-19. It is intended to be used in self-testing., Specimen:Nasal swab, Detection time at15 minutes, No need for ...
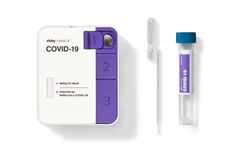
Manufactured by:Visby Medical, Inc. based inSan Jose, CALIFORNIA (USA)
Fast, accurate, and actionable PCR results in under 30 minutes. Instrument-free PCR: Empower on-demand testing, when and where you need ...
Manufactured by:Copan Diagnostics Inc. based inMurrieta, CALIFORNIA (USA)
UTM®: Universal Transport Medium™ for Collection, Transport, and Preservation of Clinical Specimens for Viral Molecular Diagnostic Testing ...
Manufactured by:Pathnostics based inIrvine, CALIFORNIA (USA)
The Pathnostics COVID-19 PCR Test detects the presence of SARS-CoV-2, the virus that causes COVID-19. The Pathnostics COVID-19 PCR Test is not impacted by the emerging B.1.1.529 (Omicron variant), enabling accurate test ...
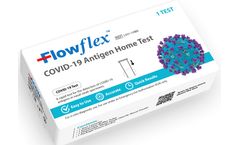
Manufactured by:ACON Laboratories, Inc. based inSan Diego, CALIFORNIA (USA)
Critical Information When & Where You Need It; The Flowflex™ COVID-19 Antigen Home Test is all you need to determine your family’s Covid-19 status, whether symptoms are present or not. Can be used on children as young as 2 years old. Get the convenience of ...
Manufactured by:Telomere Diagnostics, Inc. (TDx) based inRedwood City, CALIFORNIA (USA)
In addition to our current nasal testing offering, we are now offering saliva testing for COVID-19. Our saliva testing is a simple and pain-free process, and samples can be more easily provided as no medical personnel is required for collection. Additionally, cold shipping is not required as samples can be shipped at room ...
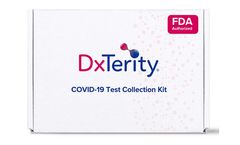
Manufactured by:DxTerity Diagnostics Inc. based inRancho Dominguez, CALIFORNIA (USA)
Our SARS-CoV-2 RT-PCR saliva-based test is FDA EUA authorized for at home unsupervised sample collection, making easy for you to take your test in the comfort of your home. Collect your sample at home and then ship it back to our lab. We use state-of-the-art technology to get your accurate results. Results are reported to you through our secure portal. ...
Manufactured by:DxTerity Diagnostics Inc. based inRancho Dominguez, CALIFORNIA (USA)
The DxTerity Immunity Assessment Test measures COVID-19 neutralizing antibody levels from a small sample of blood. Knowing your neutralizing antibody levels can help you assess your risk of future symptomatic ...
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
An immunochromatographic rapid diagnostic test (RDT) intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nasopharyngeal (NP) or anteriornasal (AN) swab specimens. Authorized for use under an Emergency Use Authorization (EUA) and for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate ...
Manufactured by:Lucira Health - Pfizer based inEmeryville, CALIFORNIA (USA)
The Lucira Check It is a molecular or nucleic acid amplification test (NAAT) that provides accurate, at-home results for the detection of the COVID-19 virus. Our tests have an analytical sensitivity (ability to detect the SARS-CoV-2 virus) that is comparable to some of the best performing PCR tests in clinical settings and ...
Manufactured by:Pathnostics based inIrvine, CALIFORNIA (USA)
The Pathnostics Respiratory Pathogens Test is a highly specific and sensitive multiplex PCR for the nucleic acid detection of SARS-Cov-2, Influenza A, Influenza B, or respiratory syncytial virus (RSV). Using one nasal swab, our Respiratory Pathogens Test will help providers diagnose patients and give appropriate therapy ...
Manufactured by:HYCOR Biomedical based inGarden Grove, CALIFORNIA (USA)
At HYCOR we are eager to help laboratories and healthcare systems by providing them with high-quality COVID-19 testing. With quick and accurate tests, patients are able to receive the healthcare and treatment they need. In response to COVID-19 outbreak, HYCOR Biomedical partners with NaGene Diagnosis who has developed the ...
Manufactured by:DxTerity Diagnostics Inc. based inRancho Dominguez, CALIFORNIA (USA)
The DxTerity SARS-CoV-2 (COVID-19) RT-PCR CE saliva-based test provides the convenience of at-home sample collection with results available within 24-72 hours. Our highly-sensitive, gold-standard PCR test has been verified to work with all COVID-19 ...
Manufactured by:Fluxergy based inIrvine, CALIFORNIA (USA)
Learn more about Fluxergy’s qualitative real-time reverse transcription polymerase chain reaction (RT-qPCR) test. For Research Use Only (RUO). Not for use in diagnostic ...
Manufactured by:Fluxergy based inIrvine, CALIFORNIA (USA)
Potential environments for rapid testing applications include emergency rooms, outpatient procedures, urgent care, workplace screening, mobile testing, community level testing settings, school reopenings, and tourism. Fluxergy’s easy-to-use qualitative RT-qPCR test can detect SARS-CoV-2 in one hour. Approved for CE-IVD. All other regions are for research use only. This device is ...
Manufactured by:Able Diagnostics, Inc. based inSan Diego, CALIFORNIA (USA)
The 1st Point-of-Care (POC) Antibody Test for COVID-19 with Fingerstick Whole Blood Authorized by FDA!. Click the link to review FDA NEWS RELEASE on Sept 23, 2020 in details: "FDA Authorizes First Point-of-Care Antibody Test for COVID-19" about Assure COVID-19 IgG/IgM Rapid Test Device. This ...
Manufactured by:DxTerity Diagnostics Inc. based inRancho Dominguez, CALIFORNIA (USA)
COVID-19 is caused by the SARS-CoV-2 virus which is a new virus in humans causing a contagious respiratory illness. COVID-19 can present with a mild to severe illness, although some people infected with COVID-19 may have no symptoms at all. Older adults and people of any age who have underlying medical conditions have a higher risk of severe illness from COVID-19. Serious outcomes of COVID-19 ...