- Home
- Equipment
- usa california
- disease risk
Refine by
Disease Risk Equipment Supplied In Usa California
7 equipment items found
Manufactured by:Pathnostics based inIrvine, CALIFORNIA (USA)
This test identifies genetic abnormalities in patients with Barrett’s esophagus and provides an indication of progression requiring additional procedures and targeted management. Utilizing cytology, immunohistochemistry (IHC), and fluorescence in situ hybridization (FISH) testing on brushings from patients with Barrett’s esophagus, patient chromosomal alterations can be distinguished ...
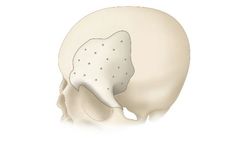
Manufactured by:Bioplate based inPlacentia, CALIFORNIA (USA)
Indicated for replacing bony voids in cranial and craniofacial skeletons, excluding maxillofacial regions, these implants avoid complications such as infection and poor blood supply, enhancing the reliability in surgical outcomes. It's crucial to avoid use in patients with systemic infections or significant degenerative diseases. Potential risks include material ...
Manufactured by:Setpoint Medical Corporation based inValencia, CALIFORNIA (USA)
SetPoint Medical created a therapy to offer patients and providers a better alternative for the treatment of chronic inflammatory diseases with potentially less risk and lower cost than drug therapy. SetPoint Medical’s bioelectronic system is free of routine injections or pills and may eliminate the risks and substantial cost associated ...
Manufactured by:Quantimetrix Corporation based inRedondo Beach, CALIFORNIA (USA)
The Lipoprint Lipoprotein Subfractions Testing System from Quantimetrix is a device for in vitro diagnostic use only, intended to measure cholesterol levels in all lipoprotein fractions and LDL subfractions in fasting serum or plasma. The Lipoprint system uses polyacrylamide gel electrophoresis to separate the various lipoprotein subfractions on the basis of size. The electrophoresed gels are ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Renasight is a test to determine if there is a genetic cause for an individual’s kidney disease or if there is an increased hereditary risk due to family history. The test uses a blood or saliva sample to test 385 genes associated with chronic kidney disease (CKD). Results are available in approximately 3 ...
Manufactured by:Quidel Corporation based inSan Diego, CALIFORNIA (USA)
Human Adenoviruses are a large group of DNA viruses that were first isolated from an adenoid gland in 1953.1 There are over 50 different serotypes.2 Adenovirus infections are often asymptomatic, but can cause a wide range of illnesses and symptoms such as: ...
Manufactured by:BioMarin based inSan Rafael, CALIFORNIA (USA)
ALDURAZYME (laronidase) is indicated for patients with Hurler and Hurler-Scheie forms of mucopolysaccharidosis I (MPS I) and for patients with the Scheie form who have moderate to severe symptoms. The risks and benefits of treating mildly affected patients with the Scheie form have not been established. ALDURAZYME has been shown to improve pulmonary function and walking capacity. ALDURAZYME has ...