- Home
- Equipment
- usa california
- drug candidate
Refine by
Drug Candidate Equipment Supplied In Usa California
14 equipment items found
Manufactured by:Addex Therapeutics based inGeneva, SWITZERLAND
Our partner, Indivior, has licensed worldwide rights to our GABAB PAM program and is responsible for all development, manufacture and commercialization of any selected GABAB PAM drug candidates. Under the agreement, we are responsible for executing a research program funded by Indivior to discover novel drug candidates. Indivior ...
Manufactured by:Addex Therapeutics based inGeneva, SWITZERLAND
Our license agreement with Indivior provides for a funded research program, under which we have the right to select drug candidates for exclusive development in certain indications outside of SUD. We plan to develop our selected drug candidates in CMT1a, chronic cough and pain. These indications have been validated with baclofen, ...
by:Ainovo Biotech Inc. based inSacramento, CALIFORNIA (USA)
AlOptimus™ brings to bear AINovo's proprietary machine learning algorithms on our highly curated datasets to enhance the therapeutic profiles of drug candidates. The AlOptimus platform optimizes validated candidates to derisk development and inform clinical ...
Manufactured by:CytomX Therapeutics, Inc. based inSouth San Francisco, CALIFORNIA (USA)
CytomX and Amgen are developing CX-904, a T-cell engaging bispecific Probody candidate against Epidermal Growth Factor Receptor (EGFR) and CD3. The drug candidate is advancing towards IND-enabling studies. CytomX is responsible for the IND filing, targeted for late 2021, and for early clinical development with Amgen leading later stage ...
Manufactured by:Addex Therapeutics based inGeneva, SWITZERLAND
Our partnered drug candidate, ADX71149 is a novel orally active mGlu2 PAM for the treatment of epilepsy. Our partner, Janssen Pharmaceuticals, Inc., or Janssen, a subsidiary of Johnson & Johnson is conducting a placebo- controlled Phase 2a proof of concept clinical trial of ADX71149 in epilepsy patients since June 2021. ...
Manufactured by:Qualigen Therapeutics, Inc. based inCarlsbad, CALIFORNIA (USA)
QN-247, formerly referred to as ALAN (Aptamer Linked Anti Nucleolin), is an aptamer-based investigational drug candidate currently under development and evaluation with the potential to treat a variety of different cancer types, including liquid and solid tumors. A key component of this drug candidate, the QN-165 aptamer, has ...
Manufactured by:Juvena Therapeutics, inc. based inRedwood City, CALIFORNIA (USA)
Secreted proteins are a proven class of biologics with tremendous therapeutic potential, as they regulate tissue repair and regeneration, organ development, and immune response to disease. The characteristics of secreted proteins render them a rich pool of drug candidates for chronic and age-related diseases. Our therapeutic approach is to unlock the potential of ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
These therapies are often associated with increased toxicity and off-target immune-related adverse effects. Unlike recombinant or engineered cytokine drug candidates, Brooklyn’s mixed cytokine product derives from human blood cells, so the cytokines have a natural conformation. This natural conformation leads to greater functionality and permits activity ...
Manufactured by:Addex Therapeutics based inGeneva, SWITZERLAND
We are developing metabotropic glutamate receptor subtype 7, or mGlu7 NAM as a novel orally available treatment to reduce fear memory in PTSD. This is a disorder that can lead to intense fear and anxiety. Current medication is unspecific and ineffective, with a number of side effects. By selectively targeting mGlu7 with NAMs, the brain circuitries involved in fear and anxiety can be more ...
Manufactured by:XILIS, Inc. based inDurham, NORTH CAROLINA (USA)
Xilis’ MicroOrganoSphereTM powers the only assay which can provide oncologists with accurate results of drug sensitivity on a patient’s own tumor within the clinical decision-making window - finally realizing the promise of precision medicine. Xilis is developing a panel of disease specific assays systematically testing the most important standard-of-care drugs within equipoise or ...
Manufactured by:ACI Medical, LLC based inSan Marcos, CALIFORNIA (USA)
The APG® Air Plethysmograph is a non-invasive diagnostic tool that quantifies the physiological components of chronic venous disease including: Chronic obstruction; Valvular reflux; Calf muscle pump function; Venous hypertension. The APG® Air Plethysmograph is the only system that measures true blood volume changes in milliliters and blood flow in milliliters per second. The APG® ...
Manufactured by:CytomX Therapeutics, Inc. based inSouth San Francisco, CALIFORNIA (USA)
Probody therapeutics are designed to outsmart cancer by exploiting the unique conditions of the tumor microenvironment to more effectively localize treatment to the tumor, while limiting activity in healthy tissues. These novel therapies are designed to take advantage of high levels of protease activity in the tumor microenvironment. Their target-binding regions are masked to limit activity ...
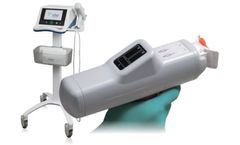
Manufactured by:Ichor Medical Systems, Inc. based inSan Diego, CALIFORNIA (USA)
Ichor® is dedicated to the clinical application and commercialization of electroporation-mediated nucleic acid delivery. Ichor’s proprietary TriGrid Delivery System uses electrical fields to significantly enhance nucleic acid delivery as compared to conventional ...
Manufactured by:Qualigen Therapeutics, Inc. based inCarlsbad, CALIFORNIA (USA)
Qualigen licensed the G4 selective transcription inhibitor platform from University College London (UCL) in 2022. The licensed technology comprises lead compound QN-302 (formerly SOP1812) and back-up compounds that target regulatory regions of cancer genes that down-regulate gene expression in multiple cancer pathways. Developed by Dr. Stephen Neidle and his group at UCL, the G4 binding concept ...