- Home
- Equipment
- usa california
- drug master file document production
Show results for
Refine by
Drug Master File Document Production Equipment Supplied In Usa California
24 equipment items found
Manufactured by:Precision NanoSystems based inVancouver, BRITISH COLUMBIA (CANADA)
Built on our revolutionary NxGen™ technology found in our NanoAssemblr® family of instruments, NanoAssemblr® GMP System enables you to go from concept to clinic with speed and confidence. Combined with PNI’s expertise, we accelerate the clinical and commercial development of your unique nanomedicine drug ...
Manufactured by:BiosPacific, Inc. based inEmeryville, CALIFORNIA (USA)
Catalog number: D57330. product description: Amikacin Polyclonal Antibody. lot number: [lot specific]. immunogen: Amikacin. source: Sheep. concentration: [lot specific] mg/mL (based on A1%. 280nm = 14.0). storage: -20°C. hazard/biohazard: There is no known biohazard associated with this ...
Manufactured by:Allergan Aesthetics based inIrvine, CALIFORNIA (USA)
Most of the SkinMedica® products described on this website are intended to meet the FDA’s definition of a cosmetic product, an article applied to the human body to cleanse, beautify, promote attractiveness, and alter appearances. These SkinMedica® products are not intended to be drug products that diagnose, treat, cure, or prevent any disease or condition. These products have not ...
by:Sutro Biopharma Inc. based inSouth San Francisco, CALIFORNIA (USA)
Sutro’s cGMP multi-product manufacturing facility, located in San Carlos, CA, utilizes single use and stainless steel technologies for fermentation, cell-free expression, and purification of Drug Intermediate and Drug ...
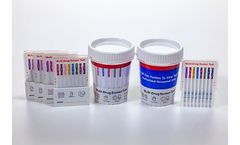
Distributed by:UCP Biosciences Inc. based inSan Jose, CALIFORNIA (USA)
Single panel u-CassettesTM can be custom configured with any CLIA waived, FDA cleared, or FUO drug tests. u-Cassettes may also be customized to include up to 6 types of adulterant tests (see tables below). ...
Distributed by:UCP Biosciences Inc. based inSan Jose, CALIFORNIA (USA)
Multi-Panel u-CassettesTM can be custom configured with any CLIA waived, FDA cleared, or FUO drug tests. u-Cassettes may also be customized to include up to 6 types of adulterant tests (see tables below). ...
Manufactured by:Coretests, Inc. based inCarlsbad, CALIFORNIA (USA)
Product Name: One-step Amphetamine Test (Cassette). Accessories: Instruction for use, pipette. Principle of Test: Immunochromatographic assay. Product Performance: Cut-off Level: ...
Manufactured by:Multiply Labs, Inc. based inSan Francisco, CALIFORNIA (USA)
Robotic system that manufacture any type of capsular ...
Manufactured by:Advin Biotech, Inc. based in, CALIFORNIA (USA)
Advin’s urine and saliva drug testing products offer premium quality and performance including the fastest results and 60 minute result stability. Flexible labeling allows us to offer generic / unbranded products or custom labeling to promote a private brand. This line of products is perfect for law enforcement, workplace testing, and insurance ...
Manufactured by:Halo Labs based inBurlingame, CALIFORNIA (USA)
If your therapy is a particle, how do you tell a good one from a bad one? The Aura CL™ system couples Fluorescent Membrane Microscopy (FMM) and Backgrounded Membrane Imaging (BMI) to help you understand what’s in your cell therapy. It detects, counts, sizes, and IDs cellular aggregates and subvisible particles and ID’s cellular from other particles without any machine learning ...
Manufactured by:BioQ Pharma based inSan Francisco, CALIFORNIA (USA)
invenious connect-and-go technology is already being used to deliver infusibles in a patented range of infusion systems. Our products are thoughtfully-designed to each drug – with bespoke solutions for: Drugs requiring complex weight-based dosing. Cytotoxic drugs. Drugs that need to be reconstituted or diluted at time of use. Drug-drug combinations, including for cold-chain ...
by:Hillhurst Biopharmaceuticals, Inc. based inSan Diego, CALIFORNIA (USA)
Therapeutic medical gases have the potential to address a wide spectrum of diseases, but have been limited by inhaled delivery, which for conscious patients is inaccurate in dosing, potentially unsafe, and highly intrusive. Hillhurst’s GLASS technology platform is designed to address the challenges of inhaled gases by enabling liquid delivery of therapeutic gases to ensure accurate dosing ...
Manufactured by:Enviroflo, Inc based inSan Jose, CALIFORNIA (USA)
The HiPo VBE is specifically designed for the weighing and manipulation of high potency powders. Independently surrogate tested to provide an ECL of <20ng/m³ when carrying out weighing and sample transfer of pharmaceutical drug ...
Manufactured by:SomaGenics, Inc. based inSanta Cruz, CALIFORNIA (USA)
RNA interference (RNAi) is a naturally occurring process by which RNA molecules inhibit gene expression either pre- or post-transcriptionally. The discovery of RNAi led to a better understanding of gene regulation and opened the door to exciting new applications in research and therapeutics. The 2006 Nobel price was awarded in recognition of this revolutionary ...
Manufactured by:ZebraSci Inc. based inTemecula, CALIFORNIA (USA)
Residual Seal Force (RSF) is a non-destructive method to evaluate seal tightness in the stopper/seal combination of parenteral vials. Parenteral vial systems consist of a vial, a stopper and an overseal. Capping parameters are a critical aspect during drug product manufacturing. During the sealing process, improper capping can generate cosmetic defects or worse yet, impact Container Closure ...
Manufactured by:Credence MedSystems, Inc. based inMenlo Park, CALIFORNIA (USA)
Pairing the right innovations creates a powerful synergy. The Companion syringe can be used as a standalone device or with the Autoinjector, giving self-injectors more flexibility while simplifying Pharma's supply chain. When a Pre-Attached Needle is preferred, the Credence Companion® Staked Needle Syringe allows the use of existing drug container components and provides integrated automatic ...
Manufactured by:Credence MedSystems, Inc. based inMenlo Park, CALIFORNIA (USA)
The Dual Chamber Reconstitution Syringe offers an enhanced user experience thanks to simplified reconstitution and injection, a pre-attached needle with passive needlestick safety, and a redefined supply chain due to the use of existing syringes and closure ...
Manufactured by:Credence MedSystems, Inc. based inMenlo Park, CALIFORNIA (USA)
When a Pre-Attached Needle is preferred, the Credence Companion® Staked Needle Syringe allows the use of existing drug container components and provides integrated automatic needle retraction and reuse prevention. The platform scales to allow 1ml Long, 2.25ml and other barrel ...
Manufactured by:Senti Biosciences based inSouth San Francisco, CALIFORNIA (USA)
Natural Killer (NK) cells are essential components of the immune system, playing an important role in the body’s defensive response against tumors and infected cells. NK cells constantly patrol the body to detect and neutralize foreign cells. Senti Bio believes there are several advantages that NK cells confer in relation to other potential immune cell types in oncology: innate killing, ...
Manufactured by:Freudenberg Medical based inCarpinteria, CALIFORNIA (USA)
A variety of specialized materials are being used in today’s MedTech environment. Many of these materials are only made suitable for the intended finished use by applying a customized surface treatment. These treatments can result in reduced friction, improvement of haptic properties, the introduction of chemical functionalities, and improved medium resistance of the materials. To meet ...