- Home
- Equipment
- usa california
- for clinical chemistry analyzers
Refine by
For Clinical Chemistry Analyzers Equipment Supplied In Usa California
22 equipment items found
Manufactured by:Carolina Liquid Chemistries Corp. based inGreensboro, NORTH CAROLINA (USA)
Carolina Liquid Chemistries provides a wide menu of reagents for use on clinical chemistry analyzers, including assays for vitamin, diabetic, renal, sepsis, stroke/inflammation, CMP, coagulation, cardiovascular/lipid, hepatic, and cancer markers. ...
Manufactured by:Carolina Liquid Chemistries Corp. based inGreensboro, NORTH CAROLINA (USA)
The best bench-top clinical chemistry analyzer on the market, this analyzer is also useful as an affordable backup, STAT lab analyzer or special chemistry analyzer. Features of a high throughput floor model built into a compact affordable bench top. Discover how your can reap ...
Manufactured by:Carolina Liquid Chemistries Corp. based inGreensboro, NORTH CAROLINA (USA)
The EasyRA benchtop analyzer is a fully automated clinical chemistry analyzer that accommodates the diverse needs of small to mid-sized laboratories and is well suited for urine drug screening and general ...
Manufactured by:Carolina Liquid Chemistries Corp. based inGreensboro, NORTH CAROLINA (USA)
The CLC800 chemistry analyzer provides cost-effective testing for small- and mid-sized laboratories. This sleek floor model fits in limited spaces to seamlessly become an integral part of your laboratory, increase productivity and provide better results. The CLC800 is quiet, small, fast and economical. The CLC800 chemistry ...
Manufactured by:Carolina Liquid Chemistries Corp. based inGreensboro, NORTH CAROLINA (USA)
Designed for large hospitals and reference labs, the FDA-cleared CLC6410 allows for high volume analysis. Expand your throughput from 1,600 tests per hour in a single module to up to 6,400 tests per hour with four modules. This model delivers a maximum ISE throughput up to 960 tests/hr and offers advanced automation features for more efficient use. The CLC6410 modular chemistry analyzer, designed ...
Manufactured by:Carolina Liquid Chemistries Corp. based inGreensboro, NORTH CAROLINA (USA)
A powerful, high-volume chemistry analyzer, the CLC1600 is perfect for both mid-size and large clinical labs. The CLC1600 offers you peace of mind with its reliable, up-to-date software, micro-electronics, fluidics and robust hardware. The efficient design increases productivity and provides better results, and is matched with the outstanding ...
Manufactured by:Siemens Healthcare GmbH based inErlangen, GERMANY
The Atellica Solution is designed to address common clinical laboratory challenges. It integratesimmunoassay and clinical chemistry analyzerswith the new standard insample-managementtechnology so you can focus on driving better ...
Manufactured by:Diatron based inBudapest, HUNGARY
The Pictus 700 is a fully automated, random access, small footprint floor clinical chemistry analyzer. The system can achieve a total throughput of up to 720 tests per ...
Manufactured by:Diatron based inBudapest, HUNGARY
The P500 is a bench top, fully automated Clinical Chemistry system with a throughput of up to 340 routine chemistry and 180 ISE tests per hour, state of the art components and user-friendly interface provide a long life platform for analytical excellence. The system includes a full range of reagents, controls and ...
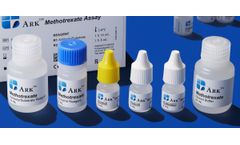
Manufactured by:ARK Diagnostics, Inc. based inFremont, CALIFORNIA (USA)
The ARK Methotrexate Assay is intended for the quantitative determination of methotrexate in human serum or plasma on automated clinical chemistry analyzers. The measurements obtained are used in monitoring levels of methotrexate to help ensure appropriate ...
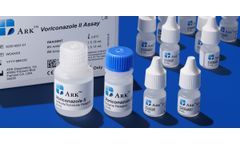
Manufactured by:ARK Diagnostics, Inc. based inFremont, CALIFORNIA (USA)
The ARK Voriconazole II Assay is intended for the quantitative determination of voriconazole in human serum on automated clinical chemistry analyzers. The measurements obtained are used in monitoring levels of voriconazole to help ensure appropriate ...
Manufactured by:ARK Diagnostics, Inc. based inFremont, CALIFORNIA (USA)
The ARK Linezolid Assay is intended for the quantitative determination of linezolid in human serum on automated clinical chemistry analyzers. The measurements obtained are used in monitoring levels of linezolid to help ensure appropriate ...
Manufactured by:ARK Diagnostics, Inc. based inFremont, CALIFORNIA (USA)
The ARK Lacosamide Assay is intended for the quantitative determination of lacosamide in human serum on automated clinical chemistry analyzers. The measurements obtained are used in monitoring levels of lacosamide to help ensure appropriate ...
Manufactured by:ARK Diagnostics, Inc. based inFremont, CALIFORNIA (USA)
The ARK™ Gabapentin Assay is intended for the quantitative determination of Gabapentin in human serum or plasma on automated clinical chemistry analyzers. Gabapentin concentrations can be used as an aid in management of patients treated with ...
Manufactured by:ARK Diagnostics, Inc. based inFremont, CALIFORNIA (USA)
The ARK™ Lamotrigine Assay is intended for the quantitative determination of Lamotrigine in human serum or plasma on automated clinical chemistry analyzers. Lamotrigine concentrations can be used as an aid in management of patients treated with ...
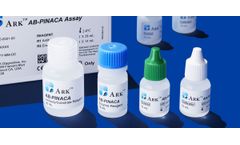
Manufactured by:ARK Diagnostics, Inc. based inFremont, CALIFORNIA (USA)
The ARK AB-PINACA Assay is an immunoassay intended for the qualitative determination of AB-PINACA and its metabolites in human urine at a cutoff concentration of 5 ng/mL. The assay is intended for use in laboratories with automated clinical chemistry ...
Manufactured by:ARK Diagnostics, Inc. based inFremont, CALIFORNIA (USA)
The ARK UR-144/JWH-018 Assay is an immunoassay intended for the qualitative determination of UR-144, JWH-018, and their metabolites in human urine at a cutoff concentration of 10 ng/mL. The assay is intended for use in laboratories with automated clinical chemistry ...
Manufactured by:ARK Diagnostics, Inc. based inFremont, CALIFORNIA (USA)
The ARK EDDP Assay is an immunoassay intended for the qualitative and/or semiquantitative determination of EDDP in human urine at cutoff concentrations of 100 ng/mL and 300 ng/mL. The assay is intended for use in laboratories with automated clinical chemistry analyzers. This in vitro diagnostic device is for prescription use ...
Manufactured by:ARK Diagnostics, Inc. based inFremont, CALIFORNIA (USA)
The ARK Efavirenz Assay is a homogeneous enzyme immunoassay intended for the quantitative determination of efavirenz in human serum or plasma on automated clinical chemistry analyzers. The serum or plasma concentration of efavirenz, used in combination with other clinical information, may aid in the management of patients treated ...
Manufactured by:Biobase LLC based inFremont, CALIFORNIA (USA)
For clinical chemistry testing: CHO, TG, HDL-C, AST, ALT, GGT, ALB, UREA, CREA ...