- Home
- Equipment
- usa california
- health organization
Show results for
Refine by
Health Organization Equipment Supplied In Usa California
11 equipment items found
Manufactured by:Arcadia Biosciences based inDavis, CALIFORNIA (USA)
ARA is an omega-6 fatty acid that is present in mother’s milk and plays a critical role in the neural and visual development of infants. ARA is one of the most abundant fatty acids in the brain, and neurological health is reliant upon sufficient levels of ARA. The use of ARA oil in infant formulas has been shown to provide developmental benefits similar to breastfeeding, ...
Manufactured by:Cornea Biosciences, Inc. based inRancho Santa Margarita, CALIFORNIA (USA)
The World Health Organization estimates over 10 million people in the world are blind in one or both eyes from corneal injury or disease and up to 45 million people could benefit from corneal transplants. According to data from eye banks and government health agencies, less than 150,000 corneal transplants are done annually worldwide due to a ...
Manufactured by:Time Medical Holding based inShatin, CHINA
The system is designed with advanced technologies such as artificial-intelligence-based deep learning algorithm with data processing system and teleradiology cloud platform, and is constructed with the latest in material technology such as thermostatic and moisture-proof materials and advanced radiation shielding technology. Incorporating advanced AI technology into medical diagnosis, the MMIS-X ...
Manufactured by:EnColl Corporation based inFremont, CALIFORNIA (USA)
The collagen used in the Hemocoll product is based on a patented technology that ensures the high purity of Type-I collagen. It is processed under ISO 13485 conditions and with stringent USP quality tests as per WHO (World Health Organization) and other international ...
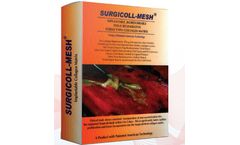
Manufactured by:EnColl Corporation based inFremont, CALIFORNIA (USA)
The collagen used in the Surgicoll-Mesh product is based on a patented technology that ensures the high purity Type-I collagen. It is processed under patented conditions and with stringent USP quality tests as per WHO (World Health Organization) and other international ...
Manufactured by:Trak Fertility based inPleasanton, CALIFORNIA (USA)
Trak is the only at home sperm test designed to help men to test, track and improve sperm count and volume over time. Other at home tests provide only “Yes” or “No” results. Trak is the only FDA cleared device that measures sperm count as Low, Moderate or Optimal for conception. Trak’s Volume Cups are the only FDA cleared way to diagnose hypospermia (low semen ...
Manufactured by:Entero Therapeutics, Inc. based inBoca Raton, FLORIDA (USA)
FW-ICI-AC is a niclosamide-based, oral, small molecule anti-inflammatory inhibitor therapy for the treatment of immune checkpoint inhibitor (ICI)-associated colitis and diarrhea in metastatic cancer patients. The PASSPORT clinical trial, a Phase 2a study to determine the safety and potential efficacy of niclosamide in the treatment of ICI-AC, received FDA IND clearance in Q4 ...
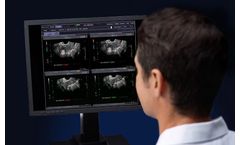
by:Imorgon Medical based inSan Mateo, CALIFORNIA (USA)
The clinical benefits of the Imorgon Ultrasound Enhancement System™ result from advanced software that fully integrates with your existing enterprise workstations and PACS. In addition, the system’s harmonization engine provides vendor-neutral compatibility with the ultrasound systems and dictation systems you’re already using. ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Covered by Medicare, Prospera is a transplant rejection assessment test that uses a simple blood draw to evaluate the risk of rejection of a transplanted kidney. Through the use of advanced cell-free DNA technology, Prospera increases a provider’s ability to identify otherwise undetected rejection that might lead to kidney loss. Catching transplant rejection as soon as possible can help ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Renasight is a test to determine if there is a genetic cause for an individual’s kidney disease or if there is an increased hereditary risk due to family history. The test uses a blood or saliva sample to test 385 genes associated with chronic kidney disease (CKD). Results are available in approximately 3 ...
Manufactured by:CoaguSense Inc. based inFremont, CALIFORNIA (USA)
The Coag-Sense PT2 Meter is designed for efficient blood coagulation monitoring, offering precise PT/INR measurements by directly measuring clot formation. By eliminating the use of look-up tables and curve fitting algorithms, the meter provides accurate prothrombin time results in less than a minute. This device is highly beneficial for both healthcare providers and patients due to its rapid and ...