- Home
- Equipment
- usa california
- heart valve
Show results for
Refine by
Heart Valve Equipment Supplied In Usa California
12 equipment items found
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
Builds upon the proven strengths of the SAPIEN valve with 5 years of durability. The SAPIEN 3 valve is the only valve approved for valve-in-valve procedures in both the aortic and mitral positions, allowing patients at high or greater surgical risk to avoid an additional open heart ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
Built on the proven SAPIEN platform, the SAPIEN 3 Ultra transcatheter heart valve was designed with patient needs in ...
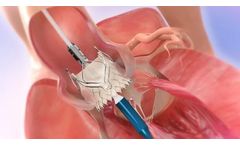
Manufactured by:JenaValve Technology, Inc. based inIrvine, CALIFORNIA (USA)
Nitinol Support Frame: Self-Expanding Design – Available in (3) Sizes. Large Cells for Coronary ...
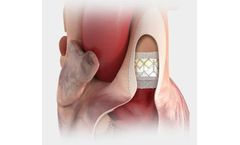
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
The first transcatheter heart valve approved for pre-stented transannular patches. Also approved for use in the pulmonic position in conduits and ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
Designed for predictable deployment. The SAPIEN XT valve may be used in the aortic position via a broad range of access routes - transfemoral, transapical and ...
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
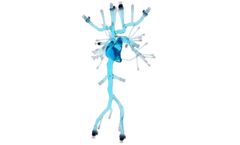
The Transcatheter Aortic Valve Replacement model is our transparent, versatile platform for training and product development. This vessel features two replaceable aortic valves, the left ventricle, and a full aorta down to the femoral arteries. Femoral access and modular heart valves allow for full simulation of transcatheter ...
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
Designed for Emerging Pulmonary Segment: Developed from DICOM Data, ensuring clinical accuracy when navigating the Right Atrium, Right Ventricle & Pulmonary Arteries. Lifelike Pulmonary Embolism (PE) & Deep Vein Thrombosis (DVT) ...
Manufactured by:JenaValve Technology, Inc. based inIrvine, CALIFORNIA (USA)
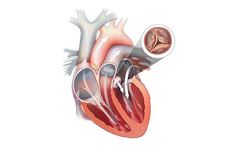
Aortic stenosis (AS) occurs when the heart’s aortic valve narrows. This narrowing prevents the valve from fully opening, which reduces or blocks blood flow from your heart into the main artery (aorta) and onward to the rest of your ...
Manufactured by:JenaValve Technology, Inc. based inIrvine, CALIFORNIA (USA)
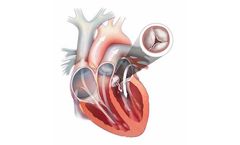
Aortic regurgitation (AR), also known as aortic insufficiency (AI), is a condition that occurs when your heart’s aortic valve doesn’t close tightly. Aortic regurgitation allows some of the blood that was pumped out of your heart’s main pumping chamber (left ventricle) to leak back into it. The leakage may prevent your ...
Manufactured by:Moray Medical based inMountain View, CALIFORNIA (USA)
Moray Medical’s initial product is the Coral System, which includes a reusable Driver Assembly, a single-use Clipper toolset, and a Trident input device. The Clipper toolset includes a cardiac-valve-therapy edge-to-edge clip implant that is pre-mounted on a single-use fluid-driven steerable delivery catheter. The Driver, a wireless capital-equipment unit about the size of a brick, ...
Manufactured by:Palisade Bio based inCarlsbad, CALIFORNIA (USA)
Our lead asset LB1148 is a novel oral liquid formulation of the well-characterized digestive enzyme inhibitor, tranexamic acid (“TXA”), with potential to both reduce abdominal adhesions and help restore bowel function following surgery. The therapy is being developed for administration prior to major surgeries that are at risk of disrupting the intestinal epithelial ...
Manufactured by:Freudenberg Medical based inCarpinteria, CALIFORNIA (USA)
Freudenberg Medical offers implantable product solutions in long-term implantable silicone material as well as implantable-grade PEEK polymers. Both long-term implantable silicone and implant-grade PEEK are suitable for long-term implantation in the human body, longer than 29 ...