- Home
- Equipment
- usa california
- medication for the treatment
Refine by
Medication For The Treatment Equipment Supplied In Usa California
19 equipment items found
Manufactured by:Stanford Advanced Materials (SAM) based inLake Forest, CALIFORNIA (USA)
Our high-quality niobium-zirconium foil widely is used in anti-corrosion, capacitors, optical target material, superconductors, medical treatment, refrigeration, ...
Manufactured by:Masimo Corp based inIrvine, CALIFORNIA (USA)
Helps reduce the symptoms of opioid withdrawal. The first FDA-cleared medical device for management of opioid withdrawal symptomsReference. Demonstrated in a clinical study to reduce withdrawal symptoms. May provide relief from opioid withdrawal symptoms, in as soon as 20 ...
by:Click Therapeutics, Inc based inNew York, NEW YORK (USA)
Clickadian is under development as a clinically-validated fully digital program for ...
by:Click Therapeutics, Inc based inNew York, NEW YORK (USA)
CT-161 is under development as a clinically-validated digital program for overactive ...
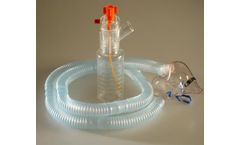
Manufactured by:B&B Medical Technologies based inCarlsbad, CALIFORNIA (USA)
Includes everything needed for high output continuous medication nebulizer delivery. The HOPE Nebulizer Kit has proven to be a cost effective solution for delivery of continuous medication for the treatment of moderate to severe life threatening asthma and COPD. The HOPE Kit is packaged with a HOPE nebulizer, adult aerosol mask, 6 feet of aerosol ...
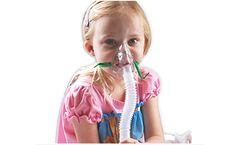
Manufactured by:B&B Medical Technologies based inCarlsbad, CALIFORNIA (USA)
Includes everything needed for high output continuous medication nebulizer delivery. The HOPE Nebulizer Kit has proven to be a cost effective solution for delivery of continuous medication for the treatment of moderate to severe life threatening asthma and COPD. The HOPE Kit is packaged with a HOPE nebulizer, pediatric aerosol mask, 6 feet of ...
Manufactured by:Synthasome Inc. based inDel Mar, CALIFORNIA (USA)
Over 4 million patients seek medical treatment each year because of a rotator cuff injury. Many of these patients will require surgery for treatment of their rotator cuff injury. Unfortunately, the failure rate for rotator cuff surgery has been reported to be between 20% and 90% in many instances. ...
by:DrKumo Inc. based inBuena Park, CALIFORNIA (USA)
Disease management programs (DMPs) are structured plans that help people manage their chronic disease, improve medical treatment in the long term, and maintain and improve quality of life. The Goals of Disease Management Programs: The main objective of Disease Management Programs is to reduce the symptoms of chronic diseases and keep them from worsening. ...
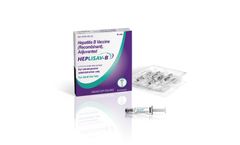
Manufactured by:Dynavax Technologies Corporation based inEmeryville, CALIFORNIA (USA)
HEPLISAV-B is indicated for prevention of infection caused by all known subtypes of hepatitis B virus in adults 18 years of age and older.1 ...
Manufactured by:NeuroSigma, Inc. based inLos Angeles, CALIFORNIA (USA)
Attention deficit hyperactivity disorder (ADHD) is one of the most commonly diagnosed neurological conditions in school-age children that often persists into adulthood. Parents and caregivers looking for alternatives to ADHD medication have a new treatment option: The Monarch eTNS (external Trigeminal Nerve Stimulation) System by ...
Manufactured by:BioMarin based inSan Rafael, CALIFORNIA (USA)
Vimizim (elosulfase alfa) is the first approved enzyme replacement therapy designed to address the underlying cause of Morquio A syndrome, or mucopolysaccharidosis IVA (MPS IVA) — a deficiency in the enzyme N-acetylgalactosamine-6 sulfatase (GALNS). VIMIZIM works at a cellular level to help with deficient enzyme ...
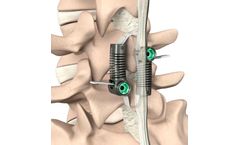
by:Empirical Spine, Inc. based inSan Carlos, CALIFORNIA (USA)
LimiFlex is intended to provide segmental stabilization following decompression for grade 1 lumbar degenerative spondylolisthesis with spinal stenosis. LimiFlex is implanted through a dorsal approach, typically through the same incision used for the surgical ...
Manufactured by:BioMarin based inSan Rafael, CALIFORNIA (USA)
The first approved treatment for any form of Batten disease. Brineura (cerliponase alfa) is indicated to slow the loss of ambulation in symptomatic pediatric patients 3 years of age and older with late infantile neuronal ceroid lipofuscinosis type 2 (CLN2), also known as tripeptidyl peptidase 1 (TPP1) deficiency. Brineura is the first enzyme replacement therapy to be directly administered into ...
Manufactured by:Biocardia, Inc. based inSunnyvale, CALIFORNIA (USA)
The investigational CardiAMP™ Cell Therapy is designed to be a comprehensive biotherapeutic heart failure solution, incorporating: a proprietary molecular diagnostic to characterize the potency of a patient’s own bone marrow cells and determine if they are an optimal candidate for therapy. a point of care processing platform to prepare cells at the patient’s bedside. an ...
Manufactured by:Nikkiso Co., Ltd. based inShibuya-ku, JAPAN
As a pioneer of dialysis machines, Nikkiso has been moving forward with the development of Hemodialysis . In order to propose a comfortable therapeutic environment for everyone who requires Hemodialysis, operations from development, manufacturing and sales, to maintenance are performed consistently at the Nikkiso Group. We promise to continue providing new proposals to customers, while making ...
Manufactured by:BioMarin based inSan Rafael, CALIFORNIA (USA)
ALDURAZYME (laronidase) is indicated for patients with Hurler and Hurler-Scheie forms of mucopolysaccharidosis I (MPS I) and for patients with the Scheie form who have moderate to severe symptoms. The risks and benefits of treating mildly affected patients with the Scheie form have not been established. ALDURAZYME has been shown to improve pulmonary function and walking capacity. ALDURAZYME has ...
Manufactured by:ACI Medical, LLC based inSan Marcos, CALIFORNIA (USA)
ArtAssist is a home-use medical treatment designed to increase blood flow without surgery so that wounds can heal and patients can keep their ...
Manufactured by:UMB Muhendislik Ltd based inEdirne, TURKEY
ARI-550 Series Medical Waste Treatment Machines specifications; Treatment method : Pre-shredder Type, Pressurized Steam Sterilization. Average Treatment Capacity : 130-150 kg / h. Feed Hopper Volume : 550 liters. Shredded Waste Part of Sterilization Vessel Volume : 290 liters. ...
Manufactured by:UMB Muhendislik Ltd based inEdirne, TURKEY
ARI-1100 Series Medical Waste Treatment Machines Specifications; Treatment method : Pre-shredder Type, Pressurized Steam Sterilization. Average Treatment Capacity : 270-400 kg / h. Feed Hopper Volume : 1100 liters. Shredded Waste Part of Sterilization Vessel Volume : 450 liters. ...