- Home
- Equipment
- usa california
- monitoring patient
Show results for
Refine by
Applications
- Remote Patient Monitoring Solution for Remote Patient Monitoring
- Remote Patient Monitoring Solution for Revenue Cycle Management
- Remote Patient Monitoring Solution for EHR Integration
- Ventilators for Heliox Ventilation
- Solutions for Glaucoma and Intraocular Pressure (IOP)
- Ventilators for Noninvasive Ventilation
- Ventilators for Nasal CPAP/CPAP+
- Ventilators for Spontaneous Positive Airway Pressure
- Ventilators for PRVC
Monitoring Patient Equipment Supplied In Usa California
96 equipment items found
Manufactured by:PAVmed Inc. based inNew York, NEW YORK (USA)
The Oncodisc device is an intelligent implantable vascular access device embedded with advanced sensor and communication technology. In addition to delivery of pharmaceutical therapy, the device reliably and accurately monitors patient physiology for early detection of illness or treatment-related complications. ...
Manufactured by:Smartizon Medical Group Limited based inArcadia, CALIFORNIA (USA)
This multi-parameter patient monitor is designed for monitoring patient's ECG, resratory rate, Temperature, Non-Invasive Blood Pressure (NIBP), pulse oxygen saturation (SpO2), Pulse Rate and other physiological ...
Manufactured by:Jant Pharmacal Corporation based inEncino, CALIFORNIA (USA)
The Coag-Sense Prothrombin Time (PT)/INR Monitoring System is an in vitro diagnostic device that provides quantitative prothrombin time (PT) results, expressed in seconds and INR units. It uses fresh capillary whole blood. It is intended for use by health care professionals at the point of care to monitor patients who are on warfarin-type ...
Manufactured by:Smartizon Medical Group Limited based inArcadia, CALIFORNIA (USA)
Multi-parameter Patient Monitor may be used to monitor patient’s 6 physiological parameters: ECG, resratory rate, body temperature, non-invasive blood pressure (NIBP), pulse oxygen saturation (SpO2), and pulse ...
Manufactured by:Chronolife SAS based inParis, FRANCE
Collecting real-life data with a certified, comfortable, and 100% washable connected medical device which improves patient compliance. Keesense is a connected medical device (CE Class IIa) in the form of a t-shirt that allows real-life measurement of multiple physiological parameters. All sensors and electronics are fully integrated into the t-shirt, therefore making it very ...
by:DrKumo Inc. based inBuena Park, CALIFORNIA (USA)
Remote Patient Monitoring (RPM) or remote physiologic monitoring is the use of electronic equipment or digital technologies to gather and monitor patient-generated health data and transfer it to their healthcare provider for diagnosis, assessment and recommendations. For example, a patient ...
Manufactured by:Mizuho America, Inc. based inUnion City, CALIFORNIA (USA)
The Radiolucent Head Frame was design for better operability and accessibility. 360 Degree Rotatable feature for the Head Frame, along with Head Arm Base, Up Grade Kit for Intraoperative CT minimizes the interference with the intraoperative CR gantry by removing the head arm during patient ...
by:Persyst Development Corporation based inSolana Beach, CALIFORNIA (USA)
Persyst Advanced Review provides offline calculation and comprehensive EEG review capabilities. Advanced Review provides all of the capabilities of Clinical Review and adds the ability to perform trending and detection on records that have not been previously processed. This can be important for records received from other institutions, or for records acquired on older equipment where Persyst ...
Manufactured by:Health By Design based inNovato, CALIFORNIA (USA)
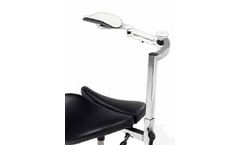
For ultrasound technicians and similar one-handed patient treatment tasks. Linear tracking arm provides support for prolonged right-arm reaching tasks. Compatible with most Salli Classic, Twin and Multiadjuster models. Chair sold separately. ...
by:Medtor Inc. based inManhattan Beach, CALIFORNIA (USA)
First ever wrist wearable , fully motion tolerant and clinical grade oxygen saturation tracking (Sp02). Accurate to FDA Requirements and low cost, ideally suited for consumer wearable and remote patient monitoring applications Custom designs are available for easy integration into your wrist wearable product. ...
Manufactured by:More Diagnostics Inc. based inLos Osos, CALIFORNIA (USA)
More Diagnostics’ Rap/Tac/CsA Control has assayed values for CEDIA® Cyclosporine /QMS® Tacrolimus (For CEDIA/QMS methods - available only from Thermo Fisher Scientific) Tacrolimus and Cyclosporine. ...
Manufactured by:Bistos Co., Ltd. based inSeongnam-si, SOUTH KOREA
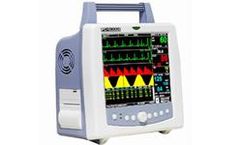
15.6" Color LCD.TFT touch screen. ECG, Resp., Sp02, NIBP, Temperature monitoring. Touch Screen/ Ultra slim design. 1S.6 color TFT touch screen ECG. Resp.. Sp02. NIBP. Temp with pacemaker detection ST segment and 16 types of arrhythmia analysis Double overpressure protection for NIBP Intelligent cuff inflation pressure adjustment Smart Hook/Stand design, provide multiple placement modes plug & ...
Manufactured by:Bistos Co., Ltd. based inSeongnam-si, SOUTH KOREA
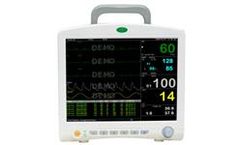
12.1' Multi-Parameter Patient Monitor ECG, Resp., Sp02, NIBP, Temperature Touch screen / Ultra slim ...
Manufactured by:Sound based inCarlsbad, CALIFORNIA (USA)
The new LOGIQ e Vet™ brings a wave of innovation to our most versatile and robust laptop ultrasound system. FEATURES: New intuitive user interface with fewer controls. Up to 90 minutes of battery life. Supports XDclear transducers. Animal patient ID screens. Optimized veterinary presets. Continuous Wave Doppler. Customizable Scan Assistant. Scan Assistant software. Patient Follow Up Tool ...
by:Persyst Development Corporation based inSolana Beach, CALIFORNIA (USA)
Persyst Clinical Review provides comprehensive universal EEG review capabilities. Clinical Review can open nearly 70 different commercial EEG formats. Regardless of the acquisition system where the recording was made, Clinical Review provides a standard, easy to use interface for reviewing EEG wave forms, Trends, Seizure Detections, and Spike Detections that have been previously processed by ...
Manufactured by:Ekso Bionics based inRichmond, CALIFORNIA (USA)
In 2013, EksoGT was released with software that adopted to patient recovery: VariableAssist. EksoGT was the only exoskeleton available for rehabilitation that could provide adaptive amounts of power to either side of the patient’s body, challenging the patient as they progress through their continuum of care, and was the first exoskeleton FDA-cleared to be used with patients of stroke and ...
Manufactured by:Pronk Technologies, Inc. based inSun Valley, CALIFORNIA (USA)
This NIBP only simulator provides features to perform basic NIBP testing of a patient monitor. Features one-button operation for quick testing of patient monitors and optional Battery Boost Option. Optional accessories provide all you need to test the NIBP parameter on any type of patient ...
Manufactured by:Tenovi based inIrvine, CALIFORNIA (USA)
Immediate Feedback: A large LCD screen clearly displays the blood glucose level. Analytics Portal: Tenovi BGM seamlessly sends data to a clinician portal for remote patient ...
Manufactured by:Biotricity Inc. based inRedwood City, CALIFORNIA (USA)
Biotres – revolutionary technology that represents the future of Remote Patient Monitoring and the delivery of real-time diagnostic data. Biotres is an easy to wear, rechargeable device with wireless capacity, leveraging the advanced technology of Bioflux® for your active patients. What does this mean for clinicians? It’s going to ...
Manufactured by:Tenovi based inIrvine, CALIFORNIA (USA)
Tenovi POx uses cellular technology to send oxygen saturation and pulse rate readings to the Tenovi cloud. Includes a real-time pulse bar graph, plethysmogram, and perfusion ...