- Home
- Equipment
- usa california
- osteoconductive
Refine by
Osteoconductive Equipment Supplied In Usa California
23 equipment items found
Manufactured by:TriMed Inc. based inSanta Clarita, CAMBODIA
A synthetic, osteoconductive and bioactive material. Manufactured by Bonalive Biomaterials Ltd in Finland from a unique S53P4 bioactive glass, characterized by its ability to firmly attach to living tissue. Indicated for bony voids or gaps that are not intrinsic to the stability of the bony ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
One of the primary functions of a bone graft material is to provide an osteoconductive surface for new bone formation. Bone regeneration is a cellular process that involves the interaction of osteoblasts and stem cells with the surface of a bone graft material. Studies have shown that the bone graft surface structure and morphology can impact how cells interact with a ...
Manufactured by:SeaSpine based inCarlsbad, CALIFORNIA (USA)
The OsteoStrand fibers were developed through a disciplined R&D process that evaluated a variety of fiber geometries to deliver osteoinductivity, osteoconductivity, intraoperative handling, and controlled expansion. OsteoStrand is 100% demineralized bone fibers designed to unleash the bone-forming capacity and fusion ...
by:Molecular Matrix, Inc. based inWest Sacramento, CALIFORNIA (USA)
Osteo-P™ Bone Graft Substitute (BGS) is a non-mineralized, synthetic bone void filler consisting of a hyper-crosslinked carbohydrate polymer. It is highly porous, biocompatible, biodegradable, and osteoconductive. Osteo-P™ BGS is intended for the filling of bone defects created surgically or as a result of traumatic injury. When placed in direct contact with patient ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
One of the primary functions of a bone graft material is to provide an osteoconductive surface for new bone formation. Bone regeneration is a cellular process that involves the interaction of osteoblasts and stem cells with the surface of a bone graft material. Studies have shown that the composition of a bone graft material can impact cellular interactions. ...
Manufactured by:SeaSpine based inCarlsbad, CALIFORNIA (USA)
OsteoStrand Plus fibers were developed through a disciplined R&D process that evaluated a variety of fiber geometries to deliver osteoinductivity, osteoconductivity, intraoperative handling, and controlled expansion. Powered by Accell® Bone Matrix (ABM), OsteoStrand Plus provides both immediate and sustained release of growth factors. OsteoStrand Plus is 100% ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
Agilon Strip Collagen Enhanced Bone Grafting Product is the latest addition to the Biogennix bone grafting product family. Developed with Biogennix proprietary TrelCor technology, Agilon Strip consists of resorbable, osteoconductive TrelCor granules densely packed in a matrix of type 1 bovine ...
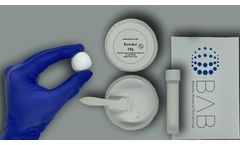
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
Bi-Ostetic Foam™ is a sterile bone graft composed of highly purified fibrillar Type I bovine collagen and Bi-Ostetic™ resorbable 60% hydroxyapatite and 40% tricalcium phosphate granules. It functions as an osteogenic stimulus to which the patient’s bone marrow can be added, prior to implantation. Bi-Ostetic Foam™ is safe and has excellent biocompatibility. After it is ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
Bi-Ostetic Bioactive Glass Foam™ is a sterile bone graft composed of highly purified fibrillar Type I bovine collagen, 45S5 bioactive glass granules and Bi-Ostetic™ resorbable 60% hydroxyapatite and 40% tricalcium phosphate granules. It functions as an osteogenic stimulus to which the patient’s bone marrow can be added to, prior to implantation. Bi-Ostetic Bioactive Glass ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
The osteoSPAN advanced bone graft product is available in a block form (in addition to granules) that was specifically designed for posterolateral spine fusion. The osteoSPAN Fusion Kit is based on the same TrelCor technology as the osteoSPAN Granules. The Fusion Kit consists of two TrelCor blocks packaged together. The osteoSPAN Fusion Kit is intended to be mixed with autograft for use as a bone ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
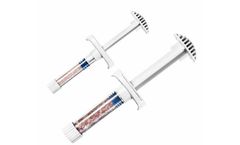
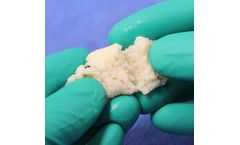
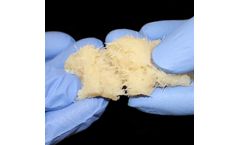
H-GENIN Crush-Mix is intended for use as a demineralized bone matrix for voids or gaps that are not intrinsic to the stability of the bony structure. Putty is indicated for the treatment of surgically created bone defects or bone defects created from traumatic injury. H-GENIN Crush-Mix provides a moldable consistency and can be mixed with blood or bone ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
H-GENIN Putty is intended for use as a demineralized bone matrix for voids or gaps that are not intrinsic to the stability of the bony structure. Putty is indicated for the treatment of surgically created bone defects or bone defects created from traumatic injury. H-GENIN Putty provides a moldable consistency and can be mixed with blood or bone ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
Cem-Ostetic® is a neutral pH bone putty that contains biocompatible calcium salts that have been used for decades in orthopedic surgery. These materials are often used to provide an additional source of bone to help the patient heal faster. Berkeley Advanced Biomaterials formulates the chemistry and microstructure to enhance bone regeneration, provide optimal osteo-conduction, and ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
ViBone is comprised of cancellous bone particles with preserved cells and demineralized cortical bone particles produced via Aziyo’s gentle proprietary VBM ...
Manufactured by:Spinal Elements, Inc. based inCarlsbad, CALIFORNIA (USA)
Viable Cell Population. An average of 1.5 million viable osteogenic cell population per cc of matrix are preserved via a non-DMSO cryoprotectant2. Endogenous bone cells remain attached via advanced processing techniques. Viable endogenous bone cells support osteogenic healing ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
Viable Bone Matrix for Spine and Orthopedic Procedures. Fiber Viable Bone Matrix: Fiber Viable Bone Matrix (VBM) is made of cancellous bone particles with preserved cells combined with demineralized cortical fiber produced via Aziyo’s gentle proprietary VBM ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
Agilon Moldable bone grafting product is the latest moldable grafting solution in Biogennix’ advanced bone graft product line. Developed with Biogennix proprietary TrelCor® technology, Agilon Moldable consists of a suspension of 1-2mm TrelCor granules in a rapidly absorbing, organic binder blended with type-1 ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
Biogennix’s flagship product brand, Morpheus, is an advanced bone graft product consisting of a moldable suspension of 1-2mm TrelCor granules in a rapidly absorbing, organic binder. Based on TrelCor technology, the granules provide enhanced bone formation due to the combination of a nanocrystalline surface with an HCA composition and interconnected pore structure that emulate human ...
Manufactured by:EnColl Corporation based inFremont, CALIFORNIA (USA)
Over 100 years of research, experimentation and use has demonstrated that calcium sulphate enhances bone regeneration and is totally biocompatible and and resorbable. At the same token, high purity type-I collagen DMBM (xenograft) also is essential for tissue regeneration and remodeling. Osseomold consists of both materials admixed in proper proportions to give the best biological and mechanical ...
Manufactured by:EnColl Corporation based inFremont, CALIFORNIA (USA)
High purity Type-I collagen derived from bone is essential for tissue regeneration and remodeling in any osseous defect. Osseograft / DMBM (xenograft) is one such de-mineralized bone derived Type-I collagen for bone void filling ...