- Home
- Equipment
- usa california
- packaging testing
Refine by
Packaging Testing Equipment Supplied In Usa California
7 equipment items found
Manufactured by:Penumbra, Inc. based inAlameda, CALIFORNIA (USA)
The Penumbra Coil 400™ (PC400™) is a large-volume platinum coil. With its larger .020″ (.508 mm) diameter, PC400 is designed to enable higher packing densities using fewer coils, leading to potential efficiencies in procedure duration, time under fluoroscopy, and treatment ...
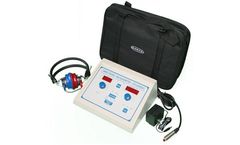
Manufactured by:AMBCO Audiometers based inTustin, CALIFORNIA (USA)
The Industry's Newest And Only Pure Tone Audiometer With Sequential ...
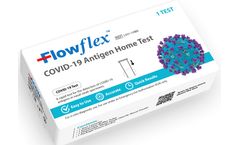
Manufactured by:ACON Laboratories, Inc. based inSan Diego, CALIFORNIA (USA)
Critical Information When & Where You Need It; The Flowflex™ COVID-19 Antigen Home Test is all you need to determine your family’s Covid-19 status, whether symptoms are present or not. Can be used on children as young as 2 years old. Get the convenience of ...
Manufactured by:CapsoVision based inSaratoga, CALIFORNIA (USA)
Developed with Silicon Valley innovation, the all-new CapsoCam Plus System is a state-of-the-art, user-friendly capsule endoscopy solution that is designed, tested, and packaged in the U.S.A. CapsoCam Plus is designed to improve diagnostic capability by providing a full 360º panoramic direct lateral view of the small bowel ...
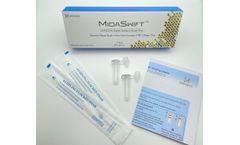
Manufactured by:Nirmidas Biotech, Inc. based inPalo Alto, CALIFORNIA (USA)
Nirmidas Biotech Inc. has developed an antigen test aiming for FDA EUA, MidaSwift™ SARS-CoV-2 Antigen Detection Kit, for the qualitative detection of the Nucleocapsid antigen from SARS-CoV-2. The test is curently for Research Use Only (RUO), for use with anterior nares (lower nostril) swab specimens. It can potentially be used at the Point-of-Care (POC) by licensed profesionals at CLIA ...
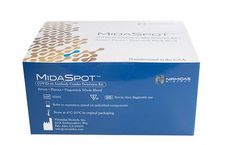
Manufactured by:Nirmidas Biotech, Inc. based inPalo Alto, CALIFORNIA (USA)
The MidaSpot COVID-19 Antibody Combo Detection Kit is a lateral flow immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in human serum, plasma (EDTA or lithium heparin), and capillary whole blood (POC fingerstick) . Nirmidas has obtained FDA EUA for this test.isting fluorescence based microplate ...
Manufactured by:Able Diagnostics, Inc. based inSan Diego, CALIFORNIA (USA)
The 1st Point-of-Care (POC) Antibody Test for COVID-19 with Fingerstick Whole Blood Authorized by FDA!. Click the link to review FDA NEWS RELEASE on Sept 23, 2020 in details: "FDA Authorizes First Point-of-Care Antibody Test for COVID-19" about Assure COVID-19 IgG/IgM Rapid Test Device. This authorization means that Fingerstick Blood Samples can now be tested in POC settings like Doctor's ...