- Home
- Equipment
- usa california
- pressure mapping sensors
Show results for
Refine by
Pressure Mapping Sensors Equipment Supplied In Usa California
12 equipment items found
Manufactured by:Alcon Management S. A. based inVernier-Geneva, SWITZERLAND
CENTURION® Vision System with ACTIVE SENTRY® is designed with advanced technology to help enhance confidence during surgery with lower, more physiological IOP and enhanced chamber stability, even in challenging ...
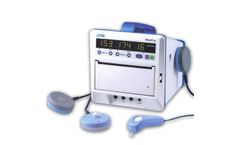
Manufactured by:Bistos Co., Ltd. based inSeongnam-si, SOUTH KOREA
It is a Antepartum Fetal Monitor measuring FHR and UC. It is a Antepartum Fetal Monitor measuring FHR and UC It enables a woman in childbed to indicate fetal movement point by pushing event marker button by herself when she feels that FM1 indicates FHR as numeral by irradiating ultrasound at the abdomen, abstracting doppler frequency of heart beat cycle from the signals reflected from fetal ...
Manufactured by:Smartizon Medical Group Limited based inArcadia, CALIFORNIA (USA)
Features: Automatic memory storage, it can store 120 units of measuring figures max. Automatic press-adding and decompressing. Manage by mmHg/kPa displaying. Turned off automatically two minutes ...
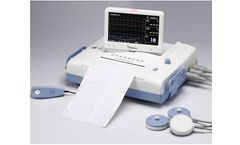
Manufactured by:Bistos Co., Ltd. based inSeongnam-si, SOUTH KOREA
BT-3S0 is an Antepartum Fetal monitor displaying FHR, and FM on the LCD screen to check fetal ...
Manufactured by:Smartizon Medical Group Limited based inArcadia, CALIFORNIA (USA)
Automatic memory storage, it can store 120 units of measuring figures max. Automatic press-adding and decompressing. Manage by mmHg/kPa displaying. Turned off automatically two minutes ...
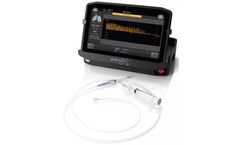
Manufactured by:Pulmonx Corporation based inRedwood City, CALIFORNIA (USA)
The Chartis® Pulmonary Assessment System is a proprietary balloon catheter and console with flow and pressure sensors used to assess the presence of collateral ventilation (CV). It is a physiological measure of collateral ventilation. ...
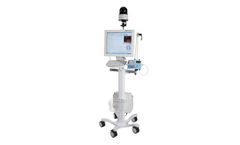
Manufactured by:Natus Medical Incorporated (Natus) based inPleasanton, CALIFORNIA (USA)
With its minimal size and medical grade all-in-one PC, the BRAIN QUICK ICU Line is ideal for EEG monitoring in the ICU and NICU. The system features an intuitive Hospital Grade, IP54 rated touch screen and the ability to set up EEG display and trend analysis in addition to event management over a network. Continuously monitor 29, 38 or 48 channels of EEG data, or combine with 2 to 17 channel ...
Manufactured by:Jan Medical, Inc. based inMountain View, CALIFORNIA (USA)
The BrainPulse system consists of a headset, data collector and computer. The headset contains a heart rate detecting sensor (PPG), a Sound Pressure Level (SPL) sensor for detecting ambient environment noise and six accelerometers to detect the acceleration of the skull at six selected locations. These components are assembled in a compact and portable headset. A touchscreen tablet provides the ...
Manufactured by:Natus Medical Incorporated (Natus) based inPleasanton, CALIFORNIA (USA)
The BRAIN QUICK Ambulatory Line can be used for in-home LTM or PSG recording and offers ease of use, patient comfort and uninterrupted data collection – critical components for home-based EEG, LTM and PSG monitoring. With 34 channels and weighing only 200 grams, the Morpheus™ recorder includes wireless intelligence to automatically switch between wired, wireless and ambulatory mode to ...
Manufactured by:Peco InspX based inBurlingame, CALIFORNIA (USA)
The Solo is the industry’s top performing, highest speed contactless rigid container inspection solution on the market. The Solo is part of Peco InspX’s family of advanced side view X-ray inspection systems and is specially designed for the inspection of rigid containers (e.g. metal and composite cans, plastic containers, glass and ...
Manufactured by:GeoEnvironment Technologies based inHouston, TEXAS (USA)
Deep well injection provides significant environmental advantages over alternative brine management options, including: Eliminating impact on surface water and shallow groundwater. Reducing or eliminating costly long-distance pipelines and ocean outfalls. Reducing surface imprint and land use impairment. Providing a sustainable local management option for urban areas and ...
Manufactured by:Penumbra, Inc. based inAlameda, CALIFORNIA (USA)
Penumbra’s Indigo Aspiration System can be used to remove emboli and thrombi from vessels of the peripheral arterial and venous systems, and for treatment of pulmonary embolism. A minimally-invasive device, Indigo enables the restoration of blood flow in such cases as acute limb ischemia and venous thrombus. The Indigo System utilizes the Penumbra ENGINE® aspiration source capable of ...