- Home
- Equipment
- usa florida
- implant for maxilofacial surgery
Refine by
Implant For Maxilofacial Surgery Equipment Supplied In Usa Florida
7 equipment items found
Manufactured by:Lenstec, Inc. based inSt Petersburg, FLORIDA (USA)
The Softec I is a C-loop, one-piece hydrophilic acrylic with a uniplanar design and equal biconvex spherical optic that allows surgeons to implant the lens whether it is right-side up or not. This makes positioning the lens virtually fool proof. The Softec I has been available globally for more than 10 years. It is part of Lenstec’s line of high value ...
Manufactured by:Clerio Vision based inNorth Sarasota, FLORIDA (USA)
Clerio Vision is developing a novel technology for writing visual corrections into intraocular lenses (IOLs) after they are implanted during cataract surgery. This unique investigational technology will offer the ability to correct the common occurrence of post-implant refractive errors, thereby non-invasively providing potentially better results and higher patient satisfaction. This technology ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Fortiva Porcine Dermis is a non-crosslinked acellular porcine dermal matrix offering a safe and natural biologic option. Fortiva implants are processed through the Tutoplast® Tissue Sterilization Process and terminally sterilized via validated low-dose gamma irradiation to achieve a sterility assurance level (SAL) of 10-6. Fortiva Porcine Dermis is a ready-to-use biologic implant that acts as ...
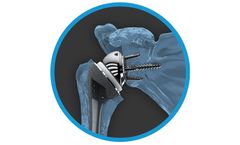
Manufactured by:Catalyst OrthoScience Inc. based inNaples, FLORIDA (USA)
The Archer R1 Reverse Shoulder System is a single-tray arthroplasty system that was designed to combine the most beneficial and evidence-based attributes of reverse shoulder arthroplasty design. The Archer R1 system offers surgeon-targeted implant positioning, a streamlined and versatile system, and bone sparing ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Cortiva Allograft Dermis is a non-crosslinked acellular dermal matrix offering a safe and natural biologic option and sterilized to a sterility assurance level (SAL) of 10-6 through the Tutoplast® Tissue Sterilization ...
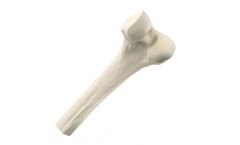
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Whole bones are used as a sterile source of cortical and cancellous bone. These implants may also be used in procedures that restore segmental and critical bone loss in the extremities due to trauma, bone disease, or from the removal of tumors and/or hardware. After being processed through RTI's BioCleanse® Tissue Sterilization Process, they are terminally sterilized in their final packaging ...
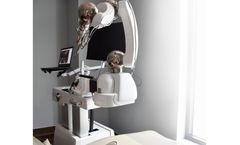
Manufactured by:Neocis, Inc. based inMiami,, FLORIDA (USA)
Yomi is the first and only FDA-cleared robotic device for dental implant surgery. Yomi provides haptic robotic guidance to augment the clinical expertise of skilled implant dentistry teams and deliver repeatable surgical precision. Yomi is in clinical use with leading dental surgeons who share the desire to elevate their dental practices and bring a new level of care to dental implant surgery. ...