- Home
- Equipment
- usa florida
- implant guided surgical
Refine by
Implant Guided Surgical Equipment Supplied In Usa Florida
13 equipment items found
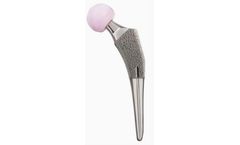
Manufactured by:Exactech, Inc based inGainesville, FLORIDA (USA)
Design features to achieve immediate axial and rotational mechanical stability. Incorporates specific design features to achieve immediate axial and rotational mechanical stability between the lateral and medial cortices of the femoral ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Non-bone tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. Non-bone tendons used in ACL reconstruction procedures allow for multiple fixation ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Matrix HD Allograft Dermis is an acellular human dermis allograft sterilized to a Sterility Assurance Level (SAL) of 10-6 using the Tutoplast Tissue Sterilization Process. This proprietary process retains the three-dimensional intertwined multidirectional fibers and mechanical properties of the native dermis tissue. The Matrix HD graft provides a natural scaffold to support the body's ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI's Achilles tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. These tendons allow for multiple techniques and fixation options in ligament reconstruction ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Conventional allografts are used for various procedures to fill bony voids or gaps in a patient's skeletal system, restore segmental bone loss due to trauma, osteoporosis, removal of tumors or to aid in the surgical correction for a deformity. These grafts provide a scaffold for bone ingrowth to allow for remodeling with the patient’s own bone. These grafts are pre-cut to select sizes to ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Fortiva Porcine Dermis is a non-crosslinked acellular porcine dermal matrix offering a safe and natural biologic option. Fortiva implants are processed through the Tutoplast® Tissue Sterilization Process and terminally sterilized via validated low-dose gamma irradiation to achieve a sterility assurance level (SAL) of 10-6. Fortiva Porcine Dermis is a ready-to-use biologic implant that acts as ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical offers the only meniscal allograft with both bone and meniscus sterilized through the BioCleanse® Tissue Sterilization Process. The BioCleanse processed meniscus graft provides a sterilized alternative to traditional aseptically processed meniscus allografts and sets the standard for safety and ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI's bone-tendon-bone (BTB) grafts are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. These grafts allow for multiple techniques and fixation options in ligament reconstruction ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Cortiva Allograft Dermis is a non-crosslinked acellular dermal matrix offering a safe and natural biologic option and sterilized to a sterility assurance level (SAL) of 10-6 through the Tutoplast® Tissue Sterilization ...
Manufactured by:Narang Medical USA Corporation based inMiami, FLORIDA (USA)
This text outlines a comprehensive range of orthopedic surgical implants and instruments specialized for the distal radius. The primary focus is on Variable Angle Volar Distal Radius Safety Lock Plates measuring 2.4mm thickness, available with various configurations, such as 4, 5, or 6 head holes, to accommodate different surgical needs. Accompanying hardware includes Safety Lock Screws which are ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
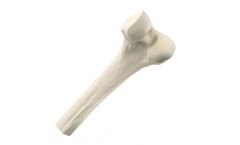
Whole bones are used as a sterile source of cortical and cancellous bone. These implants may also be used in procedures that restore segmental and critical bone loss in the extremities due to trauma, bone disease, or from the removal of tumors and/or hardware. After being processed through RTI's BioCleanse® Tissue Sterilization Process, they are terminally sterilized in their final packaging ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical’s fresh-stored osteochondral (OC) allografts enable surgeons to resurface osteochondral defects with mature hyaline cartilage and repair subchondral bone in a single procedure. Fresh grafts are cleansed, processed and preserved to maintain chondrocyte ...
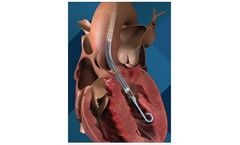
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
Impella 5.0 is a temporary, minimally invasive heart pump that provides circulatory support, enabling the heart to rest and recover. Impella LD is a surgically implanted heart pump that provides temporary support to the heart during and after a procedure. Impella 5.0 and Impella LD heart pumps allow patients to walk around while on support to improve recovery, if inserted via the axillary artery ...