- Home
- Equipment
- usa georgia
- real time micro pcr
Show results for
Refine by
Real Time Micro Pcr Equipment Supplied In Usa Georgia
5 equipment items found
Manufactured by:Omega Bio-tek, Inc. based inNorcross, GEORGIA (US) (USA)
The E.Z.N.A. Bacterial RNA Kit is designed for the isolation of high-quality total RNA from a variety of bacterial strains. Up to 1 x 109 log-phase bacterial cells can be processed. This kit uses an improved lysis procedure to ensure the complete lysis of bacterial cells. Purified RNA is suitable for downstream applications such as RT-PCR and hybridization ...
Manufactured by:Omega Bio-tek, Inc. based inNorcross, GEORGIA (US) (USA)
Omega Bio-tek’s E.Z.N.A. PX Blood RNA Kit is designed for isolation of total RNA from blood samples stored in special preserved reagents and Paxgene tubes. The procedure completely removes contaminants and enzyme inhibitors making RNA isolation fast, convenient, and reliable. RNA purified using the E.Z.N.A. PX RNA Kit method is ready for applications such as ...
Manufactured by:Omega Bio-tek, Inc. based inNorcross, GEORGIA (US) (USA)
The E.Z.N.A. HP Total RNA Isolation Kit provides a rapid and easy method for RNA isolation from cultured cells or tissues. The kit includes RNA Homogenizer columns that integrate sample homogenization and genomic DNA elimination into one single step. The RNA purification process is simplified with HiBind® Mini Column technology and can be accomplished in 25 minutes. This convenient ...
Manufactured by:OPTI Medical Systems, Inc. based inRoswell, GEORGIA (US) (USA)
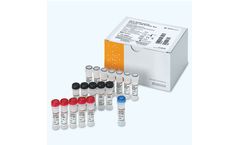
OPTI Medical Systems has obtained CE marking for its OPTI SARS-CoV-2/Influenza A/B RT-PCR Test for identification and differentiation of SARS-CoV-2, influenza A, and influenza B RNA. The test is available in countries accepting the CE mark. This respiratory panel distinguishes between SARS-CoV-2 and influenza A and B extracted from nasopharyngeal swabs. Use of this test is ideal for identifying ...
Manufactured by:OPTI Medical Systems, Inc. based inRoswell, GEORGIA (US) (USA)
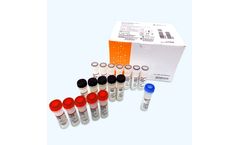
OPTI Medical Systems has received US FDA Emergency Use Authorization and CE mark for its OPTI SARS-CoV-2 RT-PCR Test for detection of the virus causing COVID-19. The OPTI SARS-CoV-2 RT-PCR Test is based on real-time reverse transcription polymerase chain reaction (RT-PCR), which provides detection of the viral RNA in the sample. The test can be used on both individual samples as well as in pools ...