- Home
- Equipment
- usa georgia
- safety product
Show results for
Refine by
Safety Product Equipment Supplied In Usa Georgia
8 equipment items found
Manufactured by:W. R. Grace & Co. based inColumbia, MARYLAND (USA)
Grace partners with customers to solve complex challenges in custom chemical synthesis and the scale up of fine chemicals for the pharmaceutical, nutraceutical, food and beverage, chemical processing, and other industries. High quality, made-to-order compounds can be manufactured in quantities ranging from kilos to tons delivered on time and upholding the highest Western safety and quality ...
Manufactured by:Owen Mumford Ltd. based inOxfordshire, UNITED KINGDOM
The next generation of safety pen needle: Unifine ?SafeControl ?is an innovative active safety pen needle designed to protect healthcare professionals and balances both safety and control during the injection ...
Manufactured by:MIMEDX based inMarietta, GEORGIA (US) (USA)
EPICORD is processed using PURION, a unique patented method for placental-based allografts that is in accordance with the American Association of Tissue Banks (AATB) standards. The product is derived from donated C-sections of live births in the US. The product undergoes active preservation of the extracellular matrix (ECM), regulatory proteins, and removal ...
Manufactured by:MIMEDX based inMarietta, GEORGIA (US) (USA)
AMNIOFIX is a dehydrated human amnion/chorion membrane allograft. The product is available in sheet, fenestrated, and wrap configurations in a variety of sizes to reduce wastage. ...
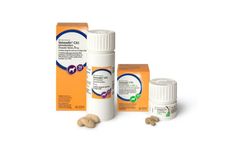
Manufactured by:Boehringer Ingelheim Animal Health USA Inc. based inDuluth, GEORGIA (US) (USA)
VETMEDIN-CA1 (pimobendan) Chewable Tablets are indicated for the delay of onset of congestive heart failure in dogs with Stage B2 preclinical myxomatous mitral valve disease (2019 American College of Veterinary Internal Medicine Consensus Statement). It is a violation of Federal law to use VETMEDIN-CA1 other than as directed in the labeling. Conditionally approved by FDA pending a full ...
Manufactured by:MIMEDX based inMarietta, GEORGIA (US) (USA)
EPIFIX is processed using PURION, a unique patented method for placental-based allografts that is in accordance with the American Association of Tissue Banks (AATB) standards. The product is derived from donated C-sections of live births in the US. The product undergoes active preservation of the extracellular matrix (ECM), regulatory proteins, and removal of ...
Manufactured by:MIMEDX based inMarietta, GEORGIA (US) (USA)
AMNIOEFFECT is a thick human amnion, intermediate layer, and chorion allograft. AMNIOEFFECT provides a semi-permeable protective barrier that supports the healing cascade and protects the wound bed to aid in the development of granulation tissue. The product is available in sheet configurations up to 180 cm2 to address a variety of surgical applications and potentially reduce ...
Manufactured by:MIMEDX based inMarietta, GEORGIA (US) (USA)
AMNIOCORD provides a protective environment to support the healing process. AMNIOCORD is derived of human umbilical cord. It is a thick graft that allows for suturing to keep the graft in place and works with deep surgical sites. ...