- Home
- Equipment
- usa illinois
- drug master file document production
Show results for
Refine by
Drug Master File Document Production Equipment Supplied In Usa Illinois
7 equipment items found
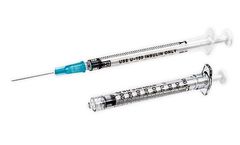
Manufactured by:Becton Dickinson and Company (BD) based inFranklin Lakes, NEW JERSEY (USA)
Our 1-mL BD Luer-Lok™ insulin syringe—available with either a detachable needle or a permanently attached needle—support many clinical uses and crucially prevent needle disengagement, medication leakage, and spray or tubing disconnect. We also offer a 1-mL insulin syringe with the BD Luer-Lok tip or the U-100 slip tip. These insulin syringes are all available at many drug ...
Manufactured by:Asahi Kasei Bioprocess based inGlenview, ILLINOIS (USA)
Planova Virus Removal Filters are specialized filtration solutions designed to provide reliable viral clearance in biopharmaceutical manufacturing. These filters, having been in use since 1989, are renowned for their robust performance and consistent quality. The series includes Planova 15N, 20N, and 35N, each offering varying pore sizes to address different virus retention requirements with high ...
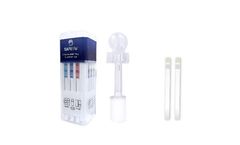
by:Wondfo USA Co Ltd. based inWillowbrook, ILLINOIS (USA)
SAFElife Oral Fluid Testing is a simple and convenient method of drug screening. Full observation during collection allows the testing to be performed anytime and anywhere, which greatly reduces specimen tamper concerns. Because it takes time for drugs to metabolize and pass through the body, oral fluid is recognized as the optimal drug screening method for recent drug ...
Manufactured by:Auris Medical AG based inBasel, SWITZERLAND
The OligoPhore™ / SemaPhore™ technology has been extensively and successfully tested with various siRNA and mRNA sequences in numerous disease models in mice. For the development of our first proprietary drug product, AM-401 (OligoPhore™ siRNA targeting KRAS), we have initiated a program of IND-enabling ...
Manufactured by:Sterigenics U.S., LLC based inOak Brook, ILLINOIS (USA)
Based on a gas diffusion process, Ethylene Oxide (EO or ETO) is capable of sterilizing and rendering products free of viable microorganisms. Sterility occurs when an EO gas molecule reacts with and destroys the microbial DNA. The process requires the simultaneous control of four variables, but interdependent parameters: gas concentration, temperature, relative humidity, and time of exposure. EO ...
Manufactured by:Sotera Health based inBroadview Heights, OHIO (USA)
Based on a gas diffusion process, Ethylene Oxide (EO or ETO) is capable of sterilizing and rendering products free of viable microorganisms. Sterility occurs when an EO gas molecule reacts with and destroys the microbial DNA. The process requires the simultaneous control of four variables, but interdependent parameters: gas concentration, temperature, relative humidity, and time of exposure. EO ...
Manufactured by:Agilent Technologies, Inc. based inSanta Clara, CALIFORNIA (USA)
BioTek Cytation 7 cell imaging multimode reader combines automated digital upright and inverted widefield microscopy with conventional multimode microplate reading. Cytation 7 includes continually variable bandpass monochromators for versatility and performance for general multimode plate reader applications. The inverted microscopy module provides sample visualization and the upright microscopy ...