- Home
- Equipment
- usa indiana
- clinical feasibility study
Show results for
Refine by
Clinical Feasibility Study Equipment Supplied In Usa Indiana
3 equipment items found
by:United Rentals Inc. based inStamford, CONNECTICUT (USA)
Pac-Van modular office buildings are truly state-of-the-art in planning and design, in choice and quality of materials, in our demanding construction standards, and in our modern installation techniques. Your temporary or permanent modular office trailer will be constructed off-site as your current site is prepared. This allows you to occupy your modular office building 30-50% sooner. Saving ...
Manufactured by:Tissue Genesis based inNew Albany, INDIANA (USA)
The Icellator2 system set the standard, providing SVF cell yields in 65 minutes. Currently approved for use in The Bahamas and available in Japan. In the USA, clinical FDA-approved IDE studies using the Icellator® are underway for a variety of ...
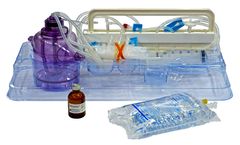
Manufactured by:Tissue Genesis based inNew Albany, INDIANA (USA)
Sterile, single-use disposable kit using adipose tissue for high efficiency enzyme derived SVF isolation with Icellator2. Functionally closed system. Minimizes process and cell product variability. Temperature controlled digestion and separation. Class III approval for commercial use by the South Korean Ministry of Food and Drug Safety (MFDS). Approved for clinical use in South Korea & The ...