- Home
- Equipment
- usa indiana
- iso 13485
Refine by
Iso 13485 Equipment Supplied In Usa Indiana
5 equipment items found
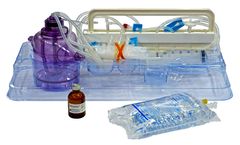
Manufactured by:Tissue Genesis based inNew Albany, INDIANA (USA)
In the USA, FDA-approved clinical IDE studies using the Icellator and Cell Icellation Kit are underway for multiple indications. Produced under ISO 13485 according to 21 CFR 820 ...
Manufactured by:Tissue Genesis based inNew Albany, INDIANA (USA)
The Icellator2 system set the standard, providing SVF cell yields in 65 minutes. Currently approved for use in The Bahamas and available in Japan. In the USA, clinical FDA-approved IDE studies using the Icellator® are underway for a variety of ...
Manufactured by:DeRoyal Industries, Inc. based inPowell, INDIANA (USA)
The STREAMWAY System installs in or on the wall with direct-to-drain fluid removal for safe, continuous collection and disposal.The illuminated touch screen provides safe control over surgical suction levels and displays automated measurement of volumes, while the single patient procedure filter and tissue trap both prevents cross contamination and allows for tissue retrieval. To clean, simply ...
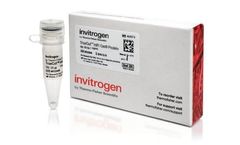
Manufactured by:Thermo Fisher Scientific based inWaltham, MASSACHUSETTS (USA)
Invitrogen TrueCut HiFi Cas9 Protein is a high fidelity CRISPR Cas9 protein variant engineered to demonstrate superior off-target profiles, while preserving maximum on-target editing efficiency. TrueCut HiFi Cas9 Protein is ideal for applications that are sensitive to off-target events and when more precise editing is ...
Manufactured by:Perma Pure LLC - a Halma Company based inLakewood, NEW JERSEY (USA)
Whether you call it moisture or humidity, dew point or condensation. Sometimes, it is essential, but often, it is a barrier to accurate breath analysis. In designing medical equipment, it is not enough to just manage moisture. You need to control it. At Perma Pure, we have worked to give you that control for the past 40 years. Perma Pure’s ME Dryers, built with Nafion™ Tubing, remove ...