- Home
- Equipment
- usa indiana
- surgery
Show results for
Refine by
Surgery Equipment Supplied In Usa Indiana
22 equipment items found
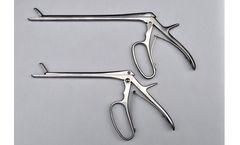
Manufactured by:White Surgical Inc. based inFort Wayne, INDIANA (USA)
Ronguers designed by surgeons for orthopedic surgery. WSKR0407 : 6MM,18CM/7” RONGEUR. WSKR0409 : 6MM,23CM/9” RONGUER. ...
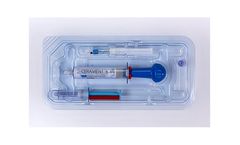
Manufactured by:OrthoPediatrics Corp. based inWarsaw, INDIANA (USA)
Calcium sulfate acts as a resorbable carrier for hydroxyapatite, and hydroxyapatite is highly osteoconductive, promoting bone ingrowth. The characteristics of CERAMENT BONE VOID FILLER make it ideal for minimally invasive surgery and open procedures where bone remodeling is ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
Zimmer Biomet’s Arcos Modular Femoral Revision System meets the demands of complex hip revision surgery by offering surgeons and OR staff the ability to customize both the hip implant and its corresponding instruments. Three proximal and five distal geometry options provide surgeons 117 proximal/distal combinations and multiple auxiliary fixation options for various femoral ...
Manufactured by:RELIGN Corporation based inWarsaw, INDIANA (USA)
Exoflow™ is RELIGN’s outflow-only fluid management offering that provides surgeons the flexibility to use their existing inflow fluid management setup while automating outflow through RELIGN devices. Exoflow features a “lock and load” self-loading cassette. The outflow activates automatically with device use as well as on-demand when the flush mode is ...
Manufactured by:White Surgical Inc. based inFort Wayne, INDIANA (USA)
Dedicated to meet your surgeon needs; Custom Boards, Pads, Pegs. Call us and tell us what you need. If we can't help you, we will point you towards someone who ...
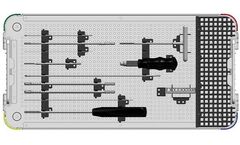
Manufactured by:Nexxt Spine, LLC based inNoblesville, INDIANA (USA)
Rod Inserter fits through Towers to align Rod straight into screw housings. Innovative approach minimizes medial and lateral soft tissue ...
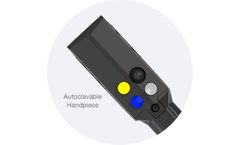
Manufactured by:RELIGN Corporation based inWarsaw, INDIANA (USA)
The Tricera Handpiece is also available in a steam autoclavable version. This handpiece provides the surgeon the same functionality and control of the procedure as the OLED version, without the visual OLED screen. This handpiece can be sterilized via steam autoclave in addition to Steris or STERRAD Hydrogen Peroxide gas sterilization. (Please refer to the Instructions for Use for detailed ...
Manufactured by:OrthoPediatrics Corp. based inWarsaw, INDIANA (USA)
OrthoPediatrics is pleased to launch the next generation Cannulated Screw System! The new system is available in multiple screw diameters including: 2.5mm, 3.0mm, 3.5mm, 4.0mm, 4.5mm and 5.5mm. For each configuration, all screws and instruments are housed in one case/tray – giving the added convenience of an “all-in-one” set. The upgraded instrumentation, and color-coding aid in ...
Manufactured by:RELIGN Corporation based inWarsaw, INDIANA (USA)
The Tricera Handpiece is an integrated bipolar RF Shaver handpiece with an OLED display. This reusable handpiece provides the surgeon exceptional control of the procedure including the ability to adjust modes, settings, motor speed, suction, and activate joint flush. The handpiece has a device recognition feature and will automatically adjust the available modes and settings for each device ...
Manufactured by:RELIGN Corporation based inWarsaw, INDIANA (USA)
RELIGN’s Tricera™ System is an innovative, integrated arthroscopic radiofrequency device, shaver, bone cutter, and fluid management system. The intuitive touchscreen display provides a quick and user-friendly setup. RELIGN’s integrated technology consolidates multiple consoles into one, minimizing space, setup, and maintenance of multiple ...
Manufactured by:OrthoPediatrics Corp. based inWarsaw, INDIANA (USA)
OrthoPediatrics has partnered with pediatric orthopedic surgeons to develop an MPFL kit to specifically address the pediatric and adolescent populations. The OrthoPediatrics’ MPFL kit allows the treating surgeon to approach each case on an individual basis, choosing the safest technique in consideration of a patient’s age, skeletal maturity, size and concomitant pathology. Regardless ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
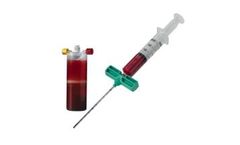
Designed to process a mixture of autologous whole blood and bone marrow aspirate, the BioCUE BBMA Concentration System represents an evolution in this technique. The system includes all the components to ASPIRATE blood and bone marrow, easily PROCESS the disposable system, and produce an autologous PRP output* to HYDRATE the surgeon’s choice of autograft and/or allograft ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
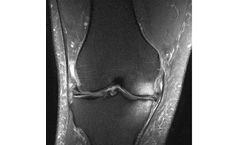
Bone Marrow Lesions (BML) are MRI-visible defects in the subchondral bone. BML can only be seen on fat-suppressed MRI sequences (T2FS, PDFS, etc.) where they appear as a hazy white area against the background of darker bone. Pathologists have shown that BML represent a healing response to trauma such as micro trabecular fractures of the subchondral ...
Manufactured by:Champion Manufacturing Inc. based inOcala, FLORIDA (USA)
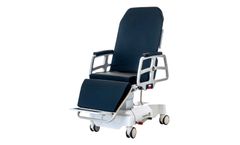
The T4 can be incorporated across a spectrum of applications and environments. With its universal appeal, it is often utilized as a procedure and/or transport chair, along with serving as a stretcher. When seconds can save lives, you need multi-purpose medical seating ...
Manufactured by:Nexxt Spine, LLC based inNoblesville, INDIANA (USA)
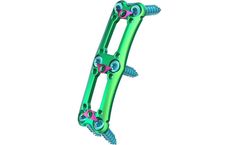
Designed for Maximum Screw Angulation to Decrease Plate Length and Potential for Adjacent Level Ossification (ALO). The Struxxure® Anterior Cervical Plate System design is based upon an emerging trend in the peer-reviewed literature identifying a significant decrease in moderate to severe ALO when the plate to disc distance is greater than 5mm from the adjacent disc level.1-5 Struxxure® ...
Manufactured by:OrthoPediatrics Corp. based inWarsaw, INDIANA (USA)
OrthoPediatricsannounces the launch of the Anterior Cruciate Ligament Reconstruction System featuring ShieldLoc and ArmorLink. The OrthoPediatrics ACL Reconstruction System is the first and only system designed with the developing patient in mind, comprised of a single system with innovative, safe instruments and implants. With the partnership of leading Pediatric Orthopedic Sports Medicine ...
Manufactured by:Nanovis based inColumbia City, INDIANA (USA)
nanoVIS Ti Surface Technology, applicable to CP Ti and Ti alloy, is a bioceramic bonegrowth nanotube surface specifically designed to increase and accelerate biologic ...
Manufactured by:Nexxt Spine, LLC based inNoblesville, INDIANA (USA)
The Blade® Anterior Cervical Plate System is intended for anterior Screw fixation during the development of cervical spine fusion. The spinal fixation device includes Self-Drilling/Self-Tapping Screws, patented ‘Hurricane’ locking mechanism, and pre-contoured 1-5 level plates. Various instruments are also available as part of the Blade® Anterior Cervical Plate System for use ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
Designed to process a mixture of autologous whole blood and bone marrow aspirate, the BioCUE BBMA Concentration System represents an evolution in this technique. The system includes all the components to DRAW blood, ASPIRATE bone marrow, easily PROCESS the disposable system, and produce an autologous PRP output to HYDRATE the surgeon’s choice of autograft and/or allograft ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
Bonus Synthetic Bone Graft Substitute granules are resorbable, osteoconductive matrices consisting of a thin, 2–10 micron layer of hydroxyapatite over a calcium carbonate core. Bonus Synthetic Bone Graft Substitute has been indicated as a bone graft substitute that resorbs and is replaced with bone during the healing ...