- Home
- Equipment
- usa maryland
- disease ttg gliadine test
Refine by
Disease Ttg Gliadine Test Equipment Supplied In Usa Maryland
5 equipment items found
Manufactured by:Novavax based inGaithersburg, MARYLAND (USA)
With the ongoing development of our NanoFlu™ and RSV F vaccines, a strong rationale exists for developing a combination respiratory vaccine that is designed to protect susceptible populations against both diseases. Although testing is at an early stage, we believe that a combination vaccine against both seasonal influenza and RSV may be ...
Manufactured by:LGC SeraCare based inMilford, MASSACHUSETTS (USA)
The Seraseq Tumor Mutation DNA Mix v2 (AF7) HC is a multiplexed mixture of actionable biosynthetic DNA targets precisely blended with a single, well-characterized genomic background. This product is produced under rigorous design control and manufacturing practices to an allelic frequency of 7%, and is offered in a high concentration (HC) format at 25 ng/µL. Designed to assess the overall ...
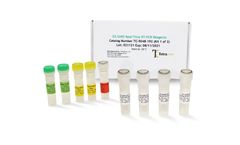
Manufactured by:Tetracore, Inc based inRockville, MARYLAND (USA)
EZ™-SARS-CoV-2 Real-Time RT-PCR is a real-time RT-PCR test intended for the qualitative detection of RNA from the SARS-CoV-2 in human nasal swab specimens from individuals suspected of COVID-19 by their healthcare provider. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high complexity tests, ...
Manufactured by:Ellume Limited based inEast Brisbane, AUSTRALIA
ellume·lab is a versatile, handheld digital device designed for healthcare professionals at the point of care. ellume·lab offers a growing range of highly accurate, rapid diagnostic tests for the detection of common infectious diseases. Two tests can be performed at the same time, with simple, animated on-screen instructions and rapid results in 15 minutes. The COVID Antigen test ...
Manufactured by:20/20 GeneSystems Inc. based inRockville, MARYLAND (USA)
20/20 GeneSystems developed PAULA’s Test, a blood test for early detection of lung cancer. It is available in the USA through licensed medical professionals. Testing is performed at 20/20’s CLIA licensed laboratory, Genesys Biolabs (CLIA Lic# 21D2037411). PAULA’s Test is meant for use in patients at high risk of lung cancer due to long term smoking. PAULA’s Test can help ...