- Home
- Equipment
- usa maryland
- phase 1 clinical
Refine by
Phase 1 Clinical Equipment Supplied In Usa Maryland
12 equipment items found
Manufactured by:Novavax based inGaithersburg, MARYLAND (USA)
In 5 separate studies, carried out in collaboration with the National Institute of Allergy and Infectious Diseases, active immunization with the Ebola GP vaccine was shown to be highly immunogenic and efficacious in preventing lethal disease in non-human primates challenged with EBOV. Our 2015 Phase 1 clinical trial demonstrated that our Ebola GP ...
Manufactured by:Novavax based inGaithersburg, MARYLAND (USA)
Older adults (aged 60 years and older) are at increased risk for RSV disease due in part to immunosenescence, the age-related decline in the human immune system. RSV infection can also lead to exacerbation of underlying comorbidities such as chronic obstructive pulmonary disease, asthma, and congestive heart failure. In the United States alone, a reported RSV incidence rate of 5.5% in older ...
Manufactured by:Elpiscience based inShanghai, CHINA
ES101 is a first-in-class tetravalent bispecific antibody targeting 4-1BB and PD-L1. It has a unique mechanism of action as 4-1BB activation is dependent on PD-L1 binding. 4-1BB is a T cell co-stimulatory receptor and is a compelling immune checkpoint target. 4-1BB targeting antibodies being developed by multinational companies have exhibited dose-dependent liver toxicity in clinical trials, ...
Manufactured by:Theriva Biologics, Inc., formely known as ynthetic Biologics, Inc. based inRockville, MARYLAND (USA)
Building on the clinical advancement of VCN-01, Theriva developed the Albumin Shield™ technology to protect oncolytic viruses from circulating anti-oncolytic virus antibodies after systemic administration. We anticipate that this technology will enable multiple oncolytic virus doses to be administered in therapeutic cycles to treat particularly refractory ...
Manufactured by:Novavax based inGaithersburg, MARYLAND (USA)
ResVax is our aluminum-adjuvanted RSV F vaccine for infants via maternal immunization. RSV is the most common cause of lower respiratory tract infections (LRTI) and the leading viral cause of severe lower respiratory tract disease in infants and young children worldwide. In the United States, RSV is the leading cause of hospitalization of infants and, globally, is second only to malaria as a ...
Manufactured by:Novavax based inGaithersburg, MARYLAND (USA)
In March 2020, we announced positive top-line results from our Phase 3 clinical trial of our nanoparticle seasonal quadrivalent influenza vaccine candidate, including our proprietary Matrix-M™ adjuvant (NanoFlu™). The trial was a randomized, observer-blinded, active-controlled trial in approximately 2,652 healthy older adults (aged 65 years and older) across 19 clinical sites in the ...
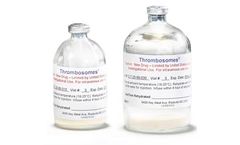
Manufactured by:Cellphire Therapeutics, Inc. based inRockville, MARYLAND (USA)
Cellphire Therapeutics is committed to transforming hemostasis management with innovative products that fill critical gaps in the treatment of bleeding patients. Our lead clinical-stage candidate, Thrombosomes, is an activated platelet-based hemostatic. The product is currently in a Phase 2 clinical trial in bleeding thrombocytopenic patients. Thrombosomes has also been given orphan drug ...
Manufactured by:Elpiscience based inShanghai, CHINA
ES002’s dual ATP-adenosine mechanism is a potentially powerful TME regulator and transformative cancer immunotherapy. In preclinical studies ES002 demonstrated significant single agent anti-tumor activity as well as an excellent tolerability profile. ES002 has also shown superior enzymatic inhibition and binding ...
Manufactured by:IDT Biologika based inDessau-Rosslau, GERMANY
IDT Biologika is a leading viral vector CDMO with an exceptional track record in biologics manufacturing, including expertise with live viral agents. This makes us well-positioned to support the rapid growth in gene therapy and immunotherapeutics markets and the huge demand for transformative viral ...
Manufactured by:Cellphire Therapeutics, Inc. based inRockville, MARYLAND (USA)
Thrombosomes are made by pooling platelet units from 10 Group O (Universal) blood donors, removing the plasma and adding a natural sugar, trehalose, to stabilize the cells. Water is then removed through a freeze-drying process resulting in a dry powder. The dry product is heat treated as a final step to reduce the risk of viral transmission and then tested for the presence of bacteria prior to ...
Manufactured by:IDT Biologika based inDessau-Rosslau, GERMANY
IDT Biologika is a leading vaccine CDMO that offers pharmaceutical companies a full range of end-to-end contract services from process development to clinical and commercial GMP manufacture of live virus drug substance at BSL-2 / BSL-3, aseptic liquid filling, lyophilization, and secondary ...
Manufactured by:Elpiscience based inShanghai, CHINA
ES104 is a bispecific antibody that simultaneously blocks Delta-like ligand 4/Notch (DLL4) and vascular endothelial growth factor A (VEGF-A) signaling pathways, which are critical to angiogenesis and tumor vascularization. VEGF also plays an important role in creating an immune suppressive TME. Pre-clinical and early clinical data of ES104 show that blocking both pathways provides robust ...