- Home
- Equipment
- usa maryland
- test patient
Refine by
Test Patient Equipment Supplied In Usa Maryland
7 equipment items found
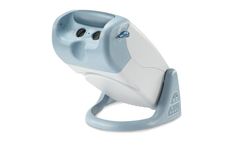
Manufactured by:Titmus, LLC based inFulton, MARYLAND (USA)
Titmus Vision Screeners are ergonomically designed, precision-built stereoscopic instruments, providing precise and prompt measurement of visual performance. The screeners are engineered for accuracy, validity and reliability with emphasis on convenience and ease of ...
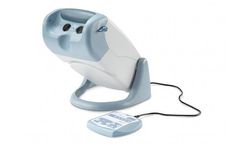
Manufactured by:Titmus, LLC based inFulton, MARYLAND (USA)
Titmus Vision Screeners are ergonomically designed, precision-built stereoscopic instruments, providing precise and prompt measurement of visual performance. The screeners are engineered for accuracy, validity and reliability with emphasis on convenience and ease of ...
Manufactured by:Ellume Limited based inEast Brisbane, AUSTRALIA
We are bringing the next generation of Latent Tuberculosis Infection diagnostics to those who need it most. In partnership with QIAGEN®, we are advancing the way tuberculosis is diagnosed. Combining our technology with Qiagen’s market leading test, QuantiFERON®, we are taking testing out of the laboratory and into the ...
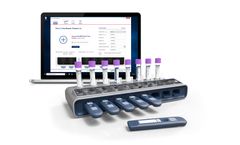
Manufactured by:Ellume Limited based inEast Brisbane, AUSTRALIA
In partnership with global diagnostics company QIAGEN®, Ellume has developed two rapid COVID-19 tests: the QIAreach™ SARS-CoV-2 Antigen Test**** for active infections and the QIAreach™ Anti-SARS-CoV-2 Total Test** for past infections. Both tests are now available in the U.S. The tests are run on the eHub platform, an easy-to-use and portable digital device that provides results in ...
Manufactured by:Emergent BioSolutions Inc. based inGaithersburg, MARYLAND (USA)
Blood glucose measurement in patients receiving VIGIV must be done with a glucose-specific method (monitor and test strips) to avoid interference by maltose contained in VIGIV. Glucose dehydrogenase pyrroloquinolinequinone (GDH-PQQ) or glucose-dye-oxidoreductase method (monitor and test strips) must not be used for blood glucose ...
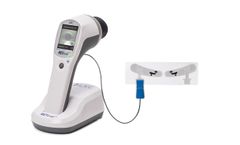
Manufactured by:LKC Technologies, Inc. based inGaithersburg, MARYLAND (USA)
The RETeval device is opening up access to functional information through visual electrophysiology testing in a whole new way. The device is the first completely portable, handheld, full field flash ERG and VEP testing device for medical professionals. Get the full picture of patient eye health with non-invasive, effective and reliable ...
Manufactured by:20/20 GeneSystems Inc. based inRockville, MARYLAND (USA)
20/20 GeneSystems developed PAULA’s Test, a blood test for early detection of lung cancer. It is available in the USA through licensed medical professionals. Testing is performed at 20/20’s CLIA licensed laboratory, Genesys Biolabs (CLIA Lic# 21D2037411). PAULA’s Test is meant for use in ...