- Home
- Equipment
- usa massachusetts
- balloon vascular catheters
Show results for
Refine by
Applications
- Embolization Device for Peripheral Vascular Disease
- Duodenal Mucosal Resurfacing System (DMR) for Type 2 Diabetes
- Duodenal Mucosal Resurfacing System (DMR) for Clinical Research Studies
- Duodenal Mucosal Resurfacing System (DMR) for T2Di Study
- Duodenal Mucosal Resurfacing System (DMR) for Healthcare Professionals
- Embolization Device for Hemorrhagic Stroke
Balloon Vascular Catheters Equipment Supplied In Usa Massachusetts
9 equipment items found
Manufactured by:SRS Medical based inN. Billerica, MASSACHUSETTS (USA)
Knowing when to remove a Foley has always been a challenge. Doing it without disturbing the catheter was impossible… until now. Attaching directly to an existing Foley catheter, the device works by using the catheter balloon to measure changes in vesical pressure during both the filling and emptying ...
Manufactured by:Artio Medical, Inc. based inPrairie Village, KANSAS (USA)
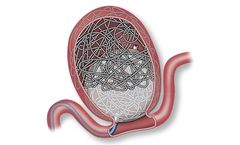
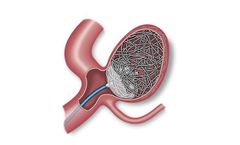
A combination of coils and an accessory balloon that has the potential to provide immediate occlusion of saccular peripheral aneurysms. Consists of an Aneura Embolization Device, Aneura Aneurysm Coils, and Aneura Balloon ...
Manufactured by:Artio Medical, Inc. based inPrairie Village, KANSAS (USA)
A combination of coils and an accessory balloon that has the potential to provide immediate cerebral aneurysm occlusion. Consists of an Endura Embolization Device, Endura Aneurysm Coils, and Endura Balloon ...
Manufactured by:Artio Medical, Inc. based inPrairie Village, KANSAS (USA)
Optimized for device deliverability and designed for occlusion of a wide range of vessel diameters and locations. The Solus Flex Embolization Device comprises a polymer balloon mounted on a dual catheter delivery system. It is paired with a device designed to anchor and support the balloon, provide a tight fit with the vessel, and resist device compression and migration. The implant is designed ...
Manufactured by:Viant Medical based inFoxborough, MASSACHUSETTS (USA)
Complex interventional devices. Steerable, balloon, and RF ablation catheters. Closure devices (femoral arterial and venous access). Introducers for transradial artery access. ...
Manufactured by:Boston Scientific based inMarlborough, MASSACHUSETTS (USA)
CRE PRO, CRE RX, CRE Fixed, and CRE Wireguided Balloon Dilatation Catheters provide consistent performance for balloon endoscopy for optimal control, efficiency and performance. Both CRE RX Biliary and PRO Wireguided Catheters are indicated for use in the removal of difficult biliary stones (Dilatation Assisted Stone Extraction, ...
Manufactured by:Boston Scientific based inMarlborough, MASSACHUSETTS (USA)
Available in a wide range of sizes, the Hurricane™ RX Biliary Balloon Dilatation Catheter is designed to reduce resistance and increase pushability when crossing ...
Manufactured by:NinePoint Medical, Inc. (NPM) based inAustin, TEXAS (USA)
The NvisionVLE Imaging System, with Real-time Targeting, as part of the VLE procedure (Volumetric Laser Endomicroscopy), uses an optical signal acquisition and processing method to create high-resolution cross-sectional images and mark tissues—letting you evaluate 100% of the tissue in a 6cm scan in real-time. 3mm deep. At a resolution of 7 microns. All so you can provide a more thorough ...
Manufactured by:Fractyl Health based inLexington, MASSACHUSETTS (USA)
Our lead product candidate, the Revita DMR System, or Revita®, is a first-in-class procedural therapy designed to target the duodenum as a root cause of type 2 diabetes. For people with advanced type 2 diabetes, the main form of therapy to control HbA1c today is insulin dose escalation and lifestyle interventions. We have developed and are investigating Revita DMR with the goal of providing ...